Drug Analysis Methods
1Institute of Biomedical Chemistry, Pogodinskaya Street, 10, Moscow 119121, Russia;
*e-mail: viktoria.shumyantseva@ibmc.msk.ru
2Pirogov Russian National Research Medical University, Ostrovitianov Street, 1, Moscow 117997, Russia
Keywords:electroanalysis; medications; diclofenac; ibuprofen; acetaminophen, erythromycin
DOI:10.18097/BMCRM00110
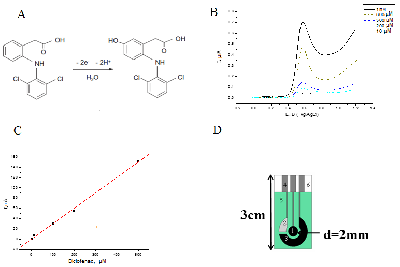
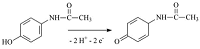
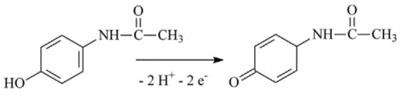
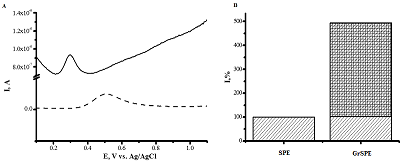
Modern methods of analysis of drugs for their quantitative assessment are considered. Particular attention is paid to the electrochemical methods of drug registration, based on the reaction of electrooxidation of molecules. Systems and materials for modifying electrodes as well as methods for producing modified electrodes for electrochemical reactions on the surface of electrodes are described . The authors present own experimental data on the electroanalysis of acetaminophen, diclofenac, ibuprofen, omeprazole, using electrodes modified with carbon nanomaterials based on carbon nanotubes and graphene. It has been shown that electroanalytical methods allow the registration of therapeutic drugs in a wide range of detectable concentrations (0.1 М - 10 mM), which can be used in biological fluid analysis(plasma, blood, urine) to conduct drug monitoring and study drug-drug interactions.
|
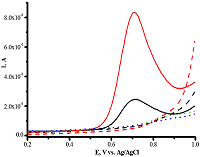
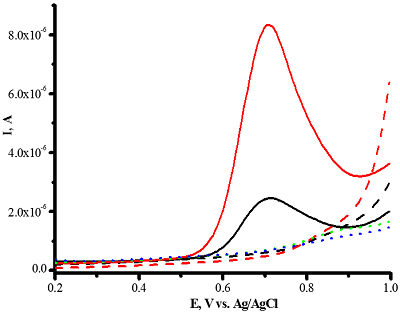
Figure 10.
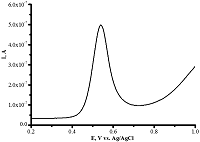
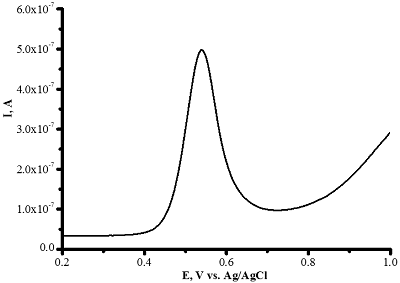
Differential pulse voltammetry of omeprazole 200 μM (-) and 1 mM (-) omeprazole using CNT/PGE (Shumyantseva V.V. et al., manuscript in preparation).
|
|
CLOSE

|
Table 1.
Electroanalytic characteristics of drugs.
|
FUNDING
The work was performed in the framework of the Program for Basic Research of State Academies of Sciences for 2013- 2020 and it was supported by Russian Fund of Fundamental Research (RFBR), research project No. 18-04-00374.
REFERENCES
- Sigolaeva, L.V., Bulko, T.V., Kozin, M.S., Zhang, W., Köhler, M., Romanenko, I., Yuan, J., Schacher, F.H., Pergushov, D.V., Shumyantseva, V. V. (2019) Long-term stable poly(ionic liquid)/MWCNTs inks enable enhanced surface modification for electrooxidative detection and quantification of dsDNA. Polymer, 168, 95-103. DOI
- Passos, M., Saraiva, M. (2019) Detection in UV-visible spectrophotometry: Detectors, detection systems, and detection strategies. Measurement, 135, 896-904.
- De Souza, M., Kogawa, A., Salgado, H. (2019) New and miniaturized method for analysis of enrofloxacin in palatable tablets. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 209, 1-7. DOI
- Duan, Q., Ma, Y., Che, M., Zhang, B., Zhang, Y., Li, Y., Zang, W., Sang, S. (2019) Fluorescent carbon dots as carriers for intracellular doxorubicin delivery and track. Journal of Drug Delivery Science and Technology, 49, 527-533.
- Guimarães, D., Noro, J., Loureiro, A., Cavaco-Paulo, A., Nogueira, E. (2019) Quantification of drugs encapsulated in liposomes by H NMR. Colloids and Surfaces B: Biointerfaces. Colloids Surf B Biointerfaces, 179, 414-420. DOI
- Hosny, N., Huddersman, K., El-Gizawy, S., Atia, N. (2019) New approach for simultaneous analysis of commonly used antigout drugs by HPLC/UV detection; Application in pharmaceutical and biological analysis. Microchemical Journal, 147, 717-728. DOI
- Kazakevich, Y., LoBrutto, R. HPLC for pharmaceutical scientists, Kazakevich, Y., Rosario LoBrutto, R., Editor. 2007, John Wiley & Sons: New Jersey, p. 1135.
- Ahuja, S., Dong, M.W. Handbook of pharmaceutical analysis by HPLC, Ahuja, S., Dong, M.W., Editor. 2005, Elsevier/ Academic Press: Amsterdam, p. 679.
- Lunn, G. HPLC methods for recently approved pharmaceuticals. 2005, John Wiley & Sons: New Jersey, p. 717. DOI
- Sona, H., Nohb, K., Kanga, C., Nac, M., Ohd, S., Songe, I., Kang, W. (2019) HPLC-MS/MS analysis of ilimaquinone and its application in apharmacokinetic study in rats. Journal of Pharmaceutical and Biomedical Analysis, 166, 291-294. DOI
- Liu, Z., Fan, H., Zhou, Y., Qian, X., Duan, G. (2019) Development and validation of a sensitive method for alkyl sulfonate genotoxic impurities determination in drug substances using gas chromatography coupled to triple quadrupole mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 168, 23-29.
- Schumacher, S., Seitz, H. (2016) A novel immunoassay for quantitative drug abuse screening in serum. Journal of Immunological Methods, 436, 34-40. DOI
- Yuan, C., Chen, D., Wang, S. (2015) Drug confirmation by mass spectrometry: Identification criteria and complicating factors. Clinica Chimica Acta, 438, 119-125. DOI
- Schulz, S., Becker, M., Groseclose, M., Schadt, S., Hopf, C. (2019) Advanced MALDI mass spectrometry imaging in pharmaceutical research and drug development. Current Opinion in Biotechnology, 55, 51-59. DOI
- Kuhlin, J., Sturkenboom, M., Ghimire, S., Margineanu, I., van den Elsen, S., Simbar, N., Akkerman, O., Jongedijk, O., Koster, R., Bruchfeld, J., Touw, D., Alffenaar, J-W. (2018). Mass spectrometry for therapeutic drug monitoring of anti-tuberculosis drugs. Clinical Mass Spectrometry, p. 12.
- Yan Peng, Lata Gautam, Sarah W. Hall, The detection of drugs of abuse and pharmaceuticals in drinking water using solid-phase extraction and liquid chromatography-mass spectrometry. Chemosphere 223 (2019) 438-447.
- Ahmad, I., Sheraz, M., Ahmed, S., Anwar, Z. (2018) Multicomponent spectrometric analysis of drugs and their preparations. Profiles of Drug Substances, Excipients and Related Methodology, 44, 379-413. DOI
- Voyeikova, T., Tyaglov, B., Antonova, S., Barsukov, E., Sizova, I., Malakhova, I., Krasikov, V. (2008) Analysis of Macrolide, Tetracyclinee and Peptide Antibiotics by Thin-Layer Chromatography. Biotechnology in Russia, 2, 105-125.
- Akhtera, S., Basiruna, W., Aliasa, Y., Johanb, M., Bagherid, S., Shalauddinb, M., Ladane, M., Anuar, N. (2018) Enhanced amperometric detection of paracetamol by immobilized cobalt ion on functionalized MWCNTs - Chitosan thin film. Analytical Biochemistry, 551, 29-36. DOI
- Shaw, L., Dennany, L. (2017). Applications of electrochemical sensors: Forensic drug analysis. Current Opinion in Electrochemistry, 3, 23-28. DOI
- Lima, H., da Silva, J., de Oliveira Farias, E., Teixeira, P., Eiras, C., Nunes, L. (2018). Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosensors and Bioelectronics, 108, 27-37. DOI
- Rahi, A., Karimian, K., Heli, H. (2016). Nanostructured materials in electroanalysis of pharmaceuticals. Analytical Biochemistry, 497, 39-47. DOI
- Cernat, A., Tertis¸ M., Sandulescu, R. (2015). Electrochemical sensors based on carbon nanomaterials for acetaminophen detection: A review. Analytica Chimica Acta, 886, 16-28. DOI
- Kutluay, A., Aslanoglu, M. (2013). Modification of electrodes using conductive porous layers to confer selectivity for the voltammetric detection of paracetamol in the presence of ascorbic acid, dopamine and uric acid. Sens. Actuat. B-Chem. 185, 398-404.
- Guo, S., Wen, D., Zhai, Y., Dong, S., Wang, E. (2011). Ionic liquid-graphene hybrid nanosheets as an enhanced material for electrochemical determination of trinitrotoluene, Biosensors and Bioelectronics, 26, 3475-3481. DOI
- Carrara, S., Cavallini, A., Erokhin, V., De Micheli, G. (2011). Multi-panel drugs detection in human serum for personalized therapy. Biosensors and Bioelectronics, 26, 3914-3919. DOI
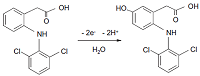
- Santini, A.O., Pezza, H.R., Pezza, L. (2006). Determination of diclofenac in pharmaceutical preparations using a potentiometric sensor immobilized in a graphite matrix. Talanta, 68(3), 636-642. DOI
- Lazarska, K., Dekker, S., Vermeulen, N., Commandeur, J. (2018). Effect of UGT2B7*2 and CYP2C8*4 polymorphisms on diclofenac metabolism. Toxicology Letters, 284, 70-78. DOI
- Goodarzian, M., Khalilzade, M., Karimi, F., Keyvanfard, V., Bagheri, H., Fouladgar, M. (2014). Square wave voltammetric determination of diclofenac in liquid phase using a novel ionic liquid multiwall carbon nanotubes paste electrode. Journal of Molecular Liquids, 197, 114-119. DOI
- Sarhangzadeh, K., Khatami, A., Jabbari, M., Bahari, S. (2013). Simultaneous determination of diclofenac and indomethacin using a sensitive electrochemical sensor based on multiwalled carbon nanotube and ionic liquid nanocomposite. J Appl Electrochem., 43, 1217-1224. DOI
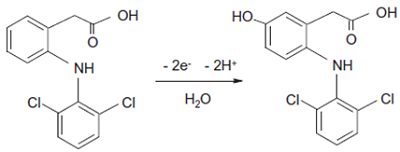
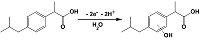
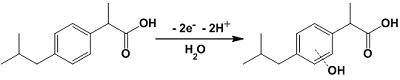
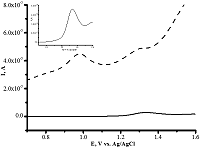
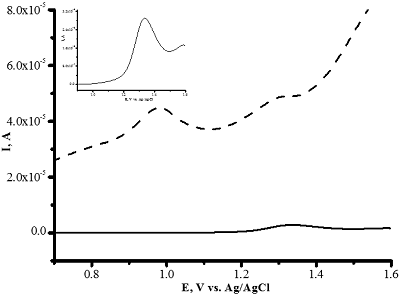
- Loudiki, A., Hammani, H., Boumya, W., Lahrich, S., Farahi, A., Achak, M., Bakasse, M., Mhammedi, M. (2016). Electrocatalytical effect of montmorillonite to oxidizing ibuprofen: Analytical application in river water and commercial tablets. Applied Clay Science, 123, 99-108. DOI
- Sugar, D., Francombe, D., da Silva T., Hanid S., Simon Hutchings, S. (2019) Comparative Bioavailability Study of a New Orodispersible Formulation of Ibuprofen Versus Two Existing Oral Tablet Formulations in Healthy Male and Female Volunteers. Clinical Therapeutics, 41, 1486-1498.
- Gasco-Lopez, A.I., Izquierdo-Hornillos, R., Jimenez, A. (1999) LC method development for ibuprophen and validation in different pharmaceuticals. Journal of Pharmaceutical and Biomedical Analysis, 21, 143-149.
- Avramov Ivic, M.L., Petrovic, S.D., Mijin, D.Z., Vanmoosa, F., Orlovic, D.Z., Marjanovic, D.Z., Radovic, V.V. (2008) The electrochemical behavior of erythromycin A on a gold electrode. Electrochimica Acta, 54, 649-654. DOI
- Hu, X., Wang, P., Yang, J., Zhang, B., Li, J., Luo, J., Wu, K. (2010). Enhanced electrochemical detection of erythromycin based on acetylene black nanoparticles. Colloids and Surfaces B: Biointerfaces, 81, 27-31. DOI
- Song, B., Zhou, Y., Jin, H., Jing, T., Zhou, T., Hao, Q., Zhou, Y., Mei, S.,Lee, Y-I. (2014). Selective and sensitive determination of erythromycin in honey and dairy products by molecularly imprinted polymers based electrochemical sensor. Microchemical Journal, 116, 183-190. DOI
- Calabresi, L., Pazzucconi, F., Ferrara, S., di Paolo, A., Del Tacca, M., Sirtori, C. (2004). Pharmacokinetic interactions between omeprazole/pantoprazole and clarithromycin in healthy volunteers. Pharmacological Research, 49, 493-499. DOI
- Bojdi, M., Behbahani, M., Mashhadizadeh, M., Bagheri, A. Davarani, S., Farahani, A. (2015). Mercapto-ordered carbohydrate-derived porous carbon electrode as a novel electrochemical sensor for simple and sensitive ultra-trace detection of omeprazole in biological samples. Materials Science and Engineering C, 48, 213-219. DOI
- Chomisteková, Z., Culková, E., Bellová, R., Melicherˆcíková, D., Durdiak, J., Timkob, J., Rievaj, M., Tomˇcík, P. (2017). Oxidation and reduction of omeprazole on boron-doped diamond electrode: Mechanistic, kinetic and sensing performance studies. Sensors and Actuators B, 241, 1194-1202. DOI
- Portychova, L., Schug, K.A. (2017). Instrumentation and applications of electrochemistry coupled to mass spectrometry for studying xenobiotic metabolism: A review. Analytica Chimica Acta, 993, 1-21. DOI
- Odijk, M., Olthuis, W., van den Berg, A., Qiao, L., Girault, H. (2012). Improved conversion rates in drug screening applications using miniaturized electrochemical cells with Frit channels. Anal. Chem., 84(21), 9176-9183. DOI
- Bisswanger, H. Enzyme kinetics. Principles and methods. (Second, revised and updated edition). 2008, WILEY-VCH Verlag GmbH & Co.: Weinheim. DOI
- Shumyantseva, V., Masamrekh, R., Kuzikov, A., Archakov, A. (2018). From electrochemistry to enzyme kinetics of cytochrome P450. Biosensors and Bioelectronics, 21, 192-204. DOI
- Arduini, F., Micheli, L., Moscone, D., Palleschi, G., Piermarini, S., Ricci, F., Volpe, G. (2016). Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. Trends in Analytical Chemistry, 79, 114-126. DOI