Preparation of Electrochemical Biosensor Systems for the Analysis of Biological Objects: a Reasonable Choice of Modifications of the Working Surface of Electrodes for Performing Research in the "Smart Electrode" Mode
1Institute of Biomedical Chemistry, 10 Pogodinskaya Street, Moscow, 119121 Russia;
*e-mail: viktoria.shumyantseva@ibmc.msk.ru
2Pirogov Russian National Research Medical University, 1 Ostrovitianova Street, Moscow, 117997 Russia
Keywords:electroanalysis; carbon nanotubes; metal nanoparticles; polymer composite materials, one- dimensional structures, biosensors
DOI:10.18097/BMCRM00119
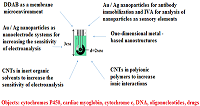
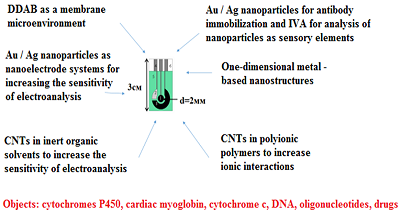
The electrochemical method of analysis of biological objects based on the reaction of electro-oxidation/electro-reduction of molecules is considered. Materials and complex systems for modifying electrodes as well as methods for producing modified electrodes to increase the sensitivity of recording the flow of electrochemical reactions on the surface of the electrodes are described. Methods of electrode modifications based on synthetic lipid-like didodecyldimethylammonium bromide, gold and silver nanoparticles, one-dimensional nanoparticles based on lead compounds, titan oxide nanoparticles, dispersions of carbon nanotubes in organic solvents, in polymers with different chemical structure are considered. It is shown that the appropriate functionalization of the working electrode surface makes it possible to increase the sensitivity of the electrochemical biosensor system and decrease the limit of detection. The results are presented in the form of an algorithm applicable for selection the beneficial type of modified electrode for the corresponding electrochemical reaction and biosample analysis.
|
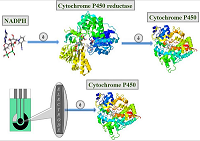
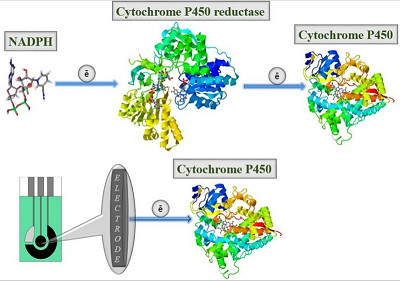
Figure 2.
Electroanalysis of cytochrome P450 using electrodes as an electron source [22].
|

|
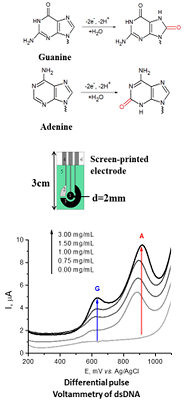
Figure 3.
Principle of electrochemical analysis of nucleic acids [11].
|
|
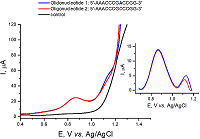
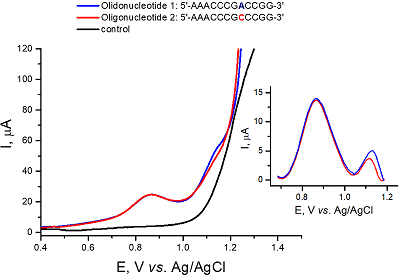
Figure 4.
Electroanalysis of oligonucleotides Oligonucleotide 1: 5’- AAACCCGACCGG–3’ and Oligonucleotide 2: 5’- AAACCCGCCCGG–3’using nanostructured electrodes [11].
|
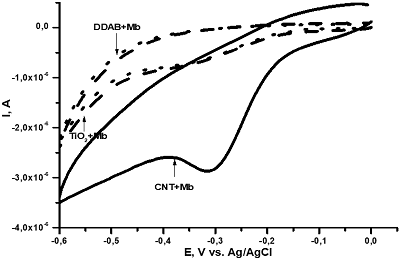
|
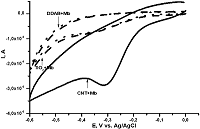
Figure 5.
Comparison of SPE/DDAB, SPE/TiO2 and SPE/MWCNT for myoglobin analysis [19].
|
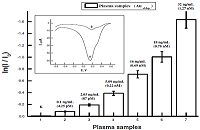
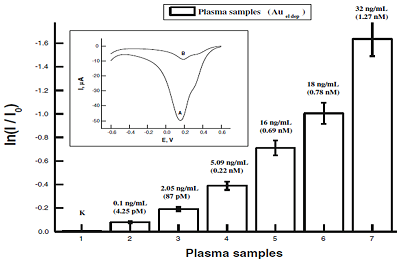
|
Figure 6.
Analysis of cardiac troponin I based on stripping voltammetry of AuNPs with detection limit of 10-10 g/ml (4.25 10-12 М). Dependence of the cathodic peak current of Auel stripping voltammetry on the amount of cTnI in plasma samples. Io corresponds to average cathodic peak current of plasma of healthy donors (HD). Inset: stripping voltammograms of SPE/AuNPel/DDAB/anticTnI+plasma of HD (A), SPE/AuNPel/DDAB/anti-cTnI+plasma of AMI (B, Acute myocardial infarction) [14].
|
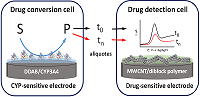
|
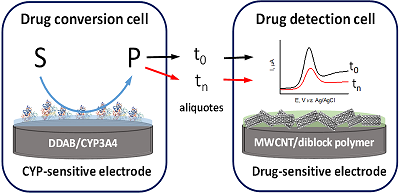
Figure 7.
Analysis of cytochrome P450 3A4 (CYP)-dependent drug metabolism [31].
|

|
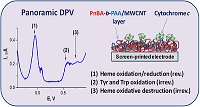
Figure 8.
Panoramic DPV as "electrochemical fingerprint" of cytochrome c deposited on a surface of SPE/PnBA100-b-PAA140/MWCNT electrode. Response for 100 µM cytochrome c with oxidation/reduction of heme (1), oxidation of amino acids Tyr + Trp (2), and electrooxidative destruction of heme (3) [13].
|
|
CLOSE

|
Table 1.
Analytical characteristics of chemically modified electrodes for the analysis of biological objects.
|
FUNDING
This work was performed within the framework of the Program for Basic Research of Russian State Academy of Sciences for 2013-2020.
REFERENCES
- Akhtera, S., Basiruna, W., Aliasa, Y., Johanb, M., Bagherid, S., Shalauddinb, M., Ladane, M., Anuar, N. (2018) Enhanced amperometric detection of paracetamol by immobilized cobalt ion on functionalized MWCNTs - Chitosan thin film. Analytical Biochemistry, 551, 29–36. DOI
- Shaw, L., Dennany, L. (2017) Applications of electrochemical sensors: Forensic drug analysis. Current Opinion in Electrochemistry, 3, 23–28. DOI
- Lima, H., da Silva, J., de Oliveira Farias, E., Teixeira, P., Eiras, C., Nunes, L. (2018) Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosensors and Bioelectronics, 108, 27–37. DOI
- Rahi, A., Karimian, K., Heli, H. (2016) Nanostructured materials in electroanalysis of pharmaceuticals. Analytical Biochemistry, 497, 39-47. DOI
- Cernat, A., Tertis¸ M., Sandulescu, R. (2015) Electrochemical sensors based on carbon nanomaterials for acetaminophen detection: A review. Analytica Chimica Acta, 886, 16-28. DOI
- Arduini, F., Micheli, L., Moscone, D., Palleschi, G., Piermarini, S., Francesco Ricci, F., Volpe, G. (2016) Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. Trends Anal. Chem., 79, 114-126. DOI
- Li, M., Li, D.-W., Xiu, G., Long, Y.-T. (2017) Applications of screen-printed electrodes in current environmental analysis. Curr. Opin. Electrochem., 3, 137-143. DOI
- Kuzikov, A.V., Bulko, T.V., Koroleva, P.I., Masamrekh, R.A., Babkina, S.S., Gilep, A.A., Shumyantseva, V.V. (2020) Cytochrome P450 3A4 as enzyme for drug biotransformation: the role of sensor systems modifications in electrocatalysis and electroanalysis. Biomeditsinskaya Khimiya, 66(1), 64-70. DOI
- Shumyantseva, V.V., Bulko, T.V., Misharin, A.Yu., Archakov, A.I. (2011) Screening of Potential Substrates or Inhibitors of Cytochrome P450 17A1 (CYP17A1) by Electrochemical Methods. Biomeditsinskaya Khimiya, 57(4), 402-409. DOI
- Shumyantseva, V.V., Bulko, T.V., Kuznetsova, G.P., Samenkova, N.F., and Archakov, A.I. (2009) Electrochemistry of cytochromes P450: Analysis of current-voltage characteristics of electrodes with immobilized cytochromes P450 for the screening of substrates and inhibitors. Biochemistry, 74, 438–444. DOI
- Sigolaeva, L.V., Bulko, T.V., Kozin, M.S., Zhang, W., Köhler, M., Romanenko, I., Yuan, J., Schacher, F.H., Pergushov, D.V., Shumyantseva, V.V. (2019) Long-term stable poly(ionic liquid)/MWCNTs inks enable enhanced surface modification for electrooxidative detection and quantification of dsDNA. Polymer, 168, 95–103. DOI
- Shumyantseva, V.V., Sigolaeva, L.V., Agafonova, L.E., Bulko, T.V., Pergushov, D.V., Schacher, F.H., Archakov, A.I. (2015) Facilitated biosensing via direct electron transfer of myoglobin integrated into diblock copolymer/multi-walled carbon nanotube nanocomposites. J. Mater. Chem. B, 3(27) 5467–5477. DOI
- Shumyantseva, V.V., Bulko, T.V., Kuzikov, A.V., Masamrekh, R.A., Pergushov, D.V., Schacher, F.H., Sigolaeva, L.V. (2020) Electrochemical fingerprint of cytochrome c on a MWCNT/polymer nanocomposite electrode. Mendeleev Communications, in press
- Shumkov, A. A., Suprun, E. V., Shatinina, S. Z., Lisitsa, A. V., Shumyantseva, V. V., Archakov, A. I. (2013) Gold and Silver Nanoparticles for Electrochemical Detection of Cardiac Troponin I based on Striping Voltammetry. BioNanoScience, 2(3), 216-222. DOI
- Shumyantseva, V.V., Bulko, T.V., Kuzikov, A.V., Masamrekh, R.A., Archakov, A.I. (2018) Analysis of L-tyrosine based on electrocatalytic oxidative reactions via screen-printed electrodes modified with multi-walled carbon nanotubes and nanosized titanium oxide (TiO2). Amino Acids., 50, 823-829. DOI
- Tong, H., Zhu, Y-J., Yang, L.X., Zhang, L. (2006) Lead Chalcogenide Nanotubes Synthesized by Biomolecule-Assisted Self-Assembly of Nanocrystals at Room Temperature. Angew. Chem. Int. Ed., 45, 7739-7742. DOI
- Shumyantseva, V. V., Bulko, T. V., Suprun, E. V. and Archakov, A. I. (2013) Electrochemical Sensor Systems Based on One_Dimensional (1D) Nanostructures for Analysis of Bioaffinity Interactions. Biomeditsinskaya Khimiya, 59(2), 209–218. DOI
- Guto, P.M., Rusling, J.F. (2006) Myoglobin retains iron heme and near-native conformation in DDAB films prepared from pH 5 to 7 dispersions. Electrochemistry Communications, 8, 455–459. DOI
- Shumyantseva, V.V., Suprun, E.V., Bulko, T.V., Dobrynina, O.V., Archakov, A.I. (2010) Sensor Systems for Medical Application Based on Hemoproteins and Nanocomposite Materials. Biomeditsinskaya Khimiya, 56 (1), 55–71. DOI
- Shumyantseva, V.V., Bulko, T.V., Suprun, E.V., Chalenko, Y.M., Vagin, M.Yu., Rudakov, Yu.O., Shatskaya, M.A., Archakov, A.I. (2011) Electrochemical investigations of cytochrome P450. Biochimica et Biophysica Acta - Proteins and Proteomics. 1814(1), 94–101. DOI
- Kuzikov, A.V., Dugin, N.O., Stulov, S.V., Shcherbinin, D.S., Zharkova, M.S., Veselovsky, A.V., Shumyantseva, V.V., Misharin, A.Y., Tkachev, Y.V., Timofeev, V.P., (2014) Novel oxazolinyl derivatives of pregna-5,17(20)-diene as 17a-hydroxylase/17,20-lyase (CYP17A1) inhibitors, Steroids, 88, 66–71. DOI
- Shumyantseva, V.V., Bulko, T.V., Suprun, E.V., Kuzikov, A.V., Agafonova, L.E., Archakov, A. I. (2015) Electrochemical Methods in Biomedical Studies. Biomeditsinskaya Khimiya, 61(2),188–202. DOI
- Shumyantseva V.V., Bulko T.V, Rudakov Yu.O., Kuznetsova G.P., Samenkova N.F., Lisitsa A.V., Karuzina I.I, Archakov A. I. (2007) Electrochemical properties of cytochroms P450 using nanostructured electrodes: Direct electron transfer and electro catalysis. J. Inorg. Biochem., 101, 859-865. DOI
- Han, X., Cheng, W., Zhang, Z., Dong, S., and Wang, E. (2002) Direct electron transfer between hemoglobin and a glassy carbon electrode facilitated by lipid-protected gold nanoparticles. Biochem. Biophys. Acta, 1556 (2-3), 273-277. DOI
- Shumyantseva, V.V., Makhova, A.A., Bulko, T.V., Kuzikov, A.V., Shich, E.V., Kukes, V., Archakov, A.I. (2015) Electrocatalytic cycle of P450 cytochromes: the protective and stimulating roles of antioxidants. RSC Advances, 5(87), 71306-71313. DOI
- Shumyantseva, V.V., Bulko, T.V., Vagin, M.Yu., Suprun, E.V., Archakov, A. I. (2010) Electrochemical Immunoanalysis of Cardiac Myoglobin. Biomeditsinskaya Khimiya, 56(6), 758–768. DOI
- Suprun, E.V., Shilovskaya, A.L., Lisitsa, A.V., Bulko, T.V. Shumyantseva, V.V., Archakov, A.I. (2011) Electrochemical Immunosensor Based on Metal Nanoparticles for Cardiac Myoglobin Detection in Human Blood Plasma. Electroanalysis, 23(5), 1051 – 1057. DOI
- Shangguan, L., Zhao, Y., Mi, L., Jiang, L, Liu, S. (2016) Direct electrochemistry and electrocatalysis of cytochrome P450s immobilized on gold/graphene-based nanocomposites. J. Electroanal. Chem., 772, 46-51. DOI
- Suprun, E.V., Bulko, T.V., Lisitsa, A.V., Gnedenko, O.V., Ivanov, A.S., Shumyantseva, V.V., Archakov, A.I. (2010) Electrochemical nanobiosensor for express diagnosis of acute myocardial infarction in undiluted plasma. Biosensors and Bioelectronics, 25 (7), 1694–1698. DOI
- Shumyantseva, V.V., Suprun, E.V., Bulko, T.V., Archakov, A.I. (2009) Electrochemical Methods for the Investigation of Bioaffinity Interactions Based on Gold Nanoparticles Modified Sensors. Electroanalysis, 21(3-5), 530 – 535. DOI
- Shumyantseva, V.V., Bulko, T.V., Kuzikov, A.V., Masamrekh, R.A., Konyakhina, A.Yu. Romanenko, I., Max, J.B., Köohler, M., Gilep, A.A., Usanov, S.A., Pergushov, D.V., Schacher, F.H., Sigolaeva, L.V. (2020) All-electrochemical nanocomposite two-electrode setup for quantification of drugs and study of their electrocatalytical conversion by cytochromes P450. Electrochimica Acta, 336, 135579. DOI
- Lin, Y-W. (2018) Structure and function of heme proteins regulated by diverse post-translational modifications. Arch. Biochem. Biophys., 641, 1-30. DOI