Аnticoronaviral activity of triterpenoids
Institute of Technical Chemistry of the Ural Branch of the RAS,
3 Academician Korolev str., Perm, 614013 Russia;
*е-mail: denisov.m@itcras.ru
Keywords:triterpenoids; coronavirus; SARS; MERS; COVID-19
DOI:10.18097/BMCRM00127
The discovery and investigations of new therapeutic agents with anticoronaviral activity is extremely important due to the COVID-19 pandemic caused by SARS-CoV-2 virus. Currently, there is no drug against COVID-19, which efficacy has been proved in correspondence with criteria of evidence-based medicine. However, there were some precursors of SARS-CoV-2 belonging to the family Coronaviridae called SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome) vuruses. Consequently, a wide range of organic substances of synthetic and natural origin were studied for the anticoronaviral activity. The review summarizes and systematizes the literature data on the anti-coronavirus activity of triterpenoids. The structural features of triterpenoids are discussed, which are important for the mechanisms of anticoronaviral activity. The structures of the most active compounds are presented. The material is classified by approaches to the study of anticoronaviral activity of individual substances or plants extracts. Recommendations for the further research of triterpenoids anticoronaviral activity are given.
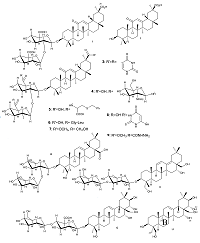
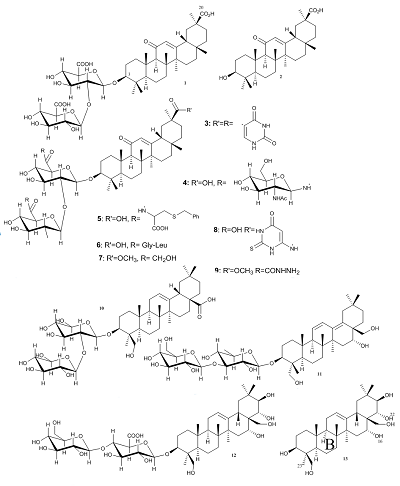
|
Figure 1.
The chemical structures of triterpenoids with tested anticoronavirus activity (compounds 1-13).
|
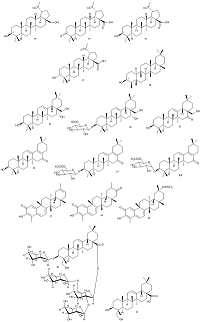
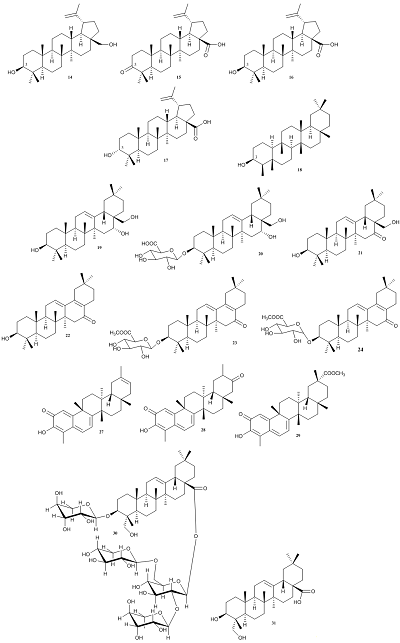
|
Figure 2.
The chemical structures of triterpenoids with tested anticoronavirus activity (compounds 14-24, 27-31).
|
|
CLOSE

|
Table 1.
Effects triterpenoids on coronaviruses replication at cell cultures.
|
|
CLOSE

|
Table 2.
Quantitative parameters of triterpenoids inhibiting the enzymatic activity of SARS-CoV 3CL protease.
|
ACKNOWLEDGEMENTS
The work was performed by the state order (project no. AAAA-A18-118030790037-7).
REFERENCES
- Vremenny’e metodicheskie rekomendaczii: profilaktika, diagnostika i lechenie novoj koronavirusnoj infekcii (COVID-19), Kamkin, E.G. Editor. 2020, Ministerstvo zdravooxraneniya rossijskoj federaczii, Moskow, 165 P. ' target='_blank' > DOI
- Ksiazek, T.G., Erdman, D., Goldsmith, C.S., Zaki, S.R., Peret, T., Emery, S., Tong, S., Urbani, C., Comer, J.A., Lim, W., Rollin, P.E., Dowell, S.F., Ling, A.E., Humphrey, C.D., Shieh, W.J., Guarner, J., Paddock, C.D., Rota, P., Fields, B., DeRisi, J., Yang, J.Y., Cox, N., Hughes, J.M., LeDuc, J.W., Bellini, W.J., Anderson, L.J. (2003) A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N Engl J Med., 348(20), 1953-1966. DOI
- Zumla, A., Hui, D.S., Perlman, S. (2015) Middle East respiratory syndrome. Lancet, 386(9997), 995-1007. DOI
- Nikiforov, V.V., Suranova, T.G., Chernobrovkina, T.Yu., Yankovskaya, Y.D., Burova S.V. (2020) New Coronavirus Infection (Covid-19): Clinical and Epidemiological Aspects, 10(2), 87-93. DOI
- Payne, S. (2017) Chapter 17 - Family Coronaviridae. Viruses, 149-158. DOI
- Li, Z., Tomlinson, A.C.A., Wong, A.H.M, Zhou, D., Desforges, M., Talbot, P.J., Benlekbir, S., Rubinstein, J.L., Rini, J.M. (2019) The human coronavirus HCoV-229E S-protein structure and receptor binding. Elife, 8, e51230-e51251. DOI
- Cheng, P.-W., Ng, L.-T., Chiang, L.-C., Lin. C.-C. (2006) Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clinical and Experimental Pharmacology and Physiology, 33(7), 612-616. DOI
- Arden, K.E., Nissen, M.D., Sloots, T.P., Mackay, I.M. (2005) New human coronavirus, HCoV‐NL63, associated with severe lower respiratory tract disease in Australia. Journal of medical virology, 75(3), 455-462. DOI
- Tsai, Y.-C., Lee, C.-L., Yen, H.-R., Chang, Y.-S., Lin, Y.-P., Huang, S.-H., Lin, C.-W. (2020) Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63 Biomolecules, 10(3), 366-373. DOI
- Vabret, A., Mourez, T., Gouarin, S., Petitjean, J., Freymuth, F. (2003) An Outbreak of Coronavirus OC43 Respiratory Infection in Normandy, France. Clinical infectious diseases, 36(8), 985-989. DOI
- Lau, S.K.P., Woo, P.C.Y., Yip, C.C.Y., Tse, H., Tsoi, H., Cheng, V.C.C., Lee, P., Tang, B.S.F., Cheung, C.H.Y., Lee, R.A., So, L., Lau, Y., Chan, K., Yuen, K. (2006) Coronavirus HKU1 and Other Coronavirus Infections in Hong Kong. J Clin Microbiol., 44(6), 2063-2071. DOI
- Kim, J.W., Ha, T.-K.-Q., Cho, H., Kim, E., Shim, S.H., Yang, J.-L., Oh W.K. (2017) Antiviral escin derivatives from the seeds of Aesculus turbinata Blume (Japanese horse chestnut). Bioorganic & Medicinal Chemistry Letters, 27(13), 3019-3025. DOI
- Baltina, L.A., Kondratenko, R.M., Baltina, L.A., Plyasunova, O.A., Pokrovskii, A.G., Tolstikov, G.A. (2009) Prospects for the creation of new antiviral drugs based on glycyrrhizic acid and its derivatives (a review). Pharmaceutical Chemistry Journal, 43(10), 539-549. DOI
- Kuo, R.-Y., Qian, K., Morris-Natschke, S.L., Lee, K.-H. (2009) Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep., 26(10), 1321-1344. DOI
- Osbourn, A., Goss, R.J.M., Field, R.A. (2011) The saponins – polar isoprenoids with important and diverse biological activities Nat. Prod. Rep., 28(7), 1261-1268. DOI
- Shang, X., Pan, H., Li, M., Miao, X., Ding, H. (2011) Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology, 138(1), 1-21. DOI
- Gupta, S., Pandotra, P., Gupta, A.P., Verma, M.K., Ahuja, A., Vishwakarma, R.A. (2013) Direct rhizogenesis, in vitro stolon proliferation and high-throughput regeneration of plantlets in Glycyrrhiza glabra. Acta Physiol Plant, 35, 2699-2705. DOI
- Xiao, S., Tian, Z., Wang, Y., Si, L., Zhang, L., Zhou, D. (2018) Recent progress in the antiviral activity andmechanism study of pentacyclic triterpenoids and their derivatives. Med Res Rev, 38(3), 951-976. DOI
- Xiaojiaoyang, L., Xiaoyu, L., Nanaa, H., Runping, L., Rong, S. (2018) A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine, 50, 73-78. DOI
- Peng, W., Liu, Y., Hu, M., Zhang, M., Yang, J., Liang, F., Huang, Q., Wu. C. (2019) Toona sinensis: a comprehensive review on its traditional usages, phytochemisty, pharmacology and toxicology. Revista Brasileira de Farmacognosia, 29, 111-124. DOI
- Batiha, G.E.-S., Beshbishy, A.M., El-Mleeh, A., Abdel-Daim, M.M., Devkota H.P. (2020) Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules, 10, 352-371. DOI
- Barnard, D.L., Kumaki, Y. (2011) Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol., 6(5), 615-631. DOI
- Retrieved May 2, 2020, from: https://www.rlsnet.ru/mnn_index_id_2448.htm
- Cinatl, J., Morgenstern, B., Bauer, G., Chandra, P., Rabenau, H., Doerr, H.W. (2003) Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet, 361, 2045-2046. DOI
- Chen, H., Du, Q. (2020) Potential natural compounds for preventing 2019-nCoV infection. Preprints, 2020010358. DOI
- Xu, X., Chen, P., Wang, J., Feng, J., Zhou, H., Li, X., Zhong, W., Hao, P. (2020) Evolution of the Novel Coronavirus From the Ongoing Wuhan Outbreak and Modeling of Its Spike Protein for Risk of Human Transmission. Sci China Life Sci., 63(3), 457-460. DOI
- Wan, Y., Shang, J., Graham, R., Baric, R.S., Li, F. (2020) Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol., 94(7), e00127-e00136. DOI
- Luo Liu, P.D., Li., J. (2020) Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. International Journal of Antimicrobial Agents, in press 105995. DOI
- Hoever, G., Baltina, L., Michaelis, M., Kondratenko, R., Baltina, L., Tolstikov, G.A., Doerr, H.W. J. Cinatl. (2005) Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS-Coronavirus. J. Med. Chem., 48, 1256-1259. DOI
- Baltina, L.A. (2003) Chemical Modification of Glycyrrhizic Acid As A Route to New Bioactive Compounds for Medicine. Current Medicinal Chemistry, 10, 155-171. DOI
- Yakovishin, L.A., Grishkovets, V.I. (2018) Molecular complexes of IVy triterpene glycosides with cholesterol. Chemistry of plant raw material, (4), 133-140. DOI
- Wu, C.-Y., Jan, J.-T., Ma, S.-H., Kuo, C.-J., Juan, H.-F., Cheng, Y.-S.E., Hsu, H.-H., Huang, H.-C., Wu, D., Brik, A., Liang, F.-S., Liu, R.-S., Fang, J.-M., Chen, S.-T., Liang, P.-H., Wong C.-H. (2004) Small molecules targeting severe acute respiratory syndrome human coronavirus. PNAS, 101(27), 10012-10017. DOI
- Petukhova, S.A., Olennikov, D.N., Mirovich, V.M. (2019) Triterpene compounds of the above ground organs of the bupleurum scorzonerifolium willd. Of the baikal region flora, (4), 215-222. DOI
- Wei, Y., Ma, C.-M., Hattori, M. (2009) Synthesis and Evaluation of A-seco Type Triterpenoids for anti-HIV-1protease Activity. Eur. J. Med. Chem., 44(10), 4112-4120. DOI
- Skvortsov, V.S., Druzhilovskiy, D.S., Veselovsky, A.V. (2020) Potential Inhibitors of Protease 3CLpro Virus COVID-19: Drug Reposition. Biomedical Chemistry: Research and Methods, 3(1), e00124-e00131. DOI
- Wen, C.-C., Kuo, Y.-H., Jan, J.-T., Liang, P.-H., Wang, S.-Y., Liu, H.-G., Lee, C.-K., Chang, X.S.-T., Kuo, C.-J., Lee, S.-S., Hou, C.-C., Hsiao, P.-W., Chien, S.-C., Shyur, L.-F., Yang, N.-S. (2007) Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem., 50, 4087-4095. DOI
- Hsu, M.F., Kuo, C.J., Chang, K.T., Chang, H.C., Chou, C.C., Ko, T.P., Shr, H.L., Chang, G.G., Wang, A.H., Liang, P.H. (2005) Mechanism of the Maturation Process of SARS-CoV 3CL Protease. Journal of Biological Chemistry, 280(35), 31257-31266. DOI
- Bureeva, S., Andia-Pravdivy, J., Symon, A., Bichucher, A., Moskaleva, V., Popenko, V., Shpak, A., Shvets, V., Kozlov, L., Kaplun, A. (2007) Selective inhibition of the interaction of C1q with immunoglobulins and the classical pathway of complement activation by steroids and triterpenoids sulfates. Bioorganic & Medicinal Chemistry, 15, 3489-3498. DOI
- Baglivo, M., Baronio, M., Natalini, G., Beccari, T., Chiurazzi, P., Fulcheri, E., Petralia, P., Michelini, S., Fiorentini, G., Miggiano, G.A., Morresi, A., Tonini, G., Bertelli, M. (2020) Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed, 91(1), 161-164. DOI
- Verma, S.P. (2009) HIV: A Raft-Targeting Approach for Prevention and Therapy Using Plant-Derived Compounds (Review). Curr Drug Targets, 10(1), 51-59. DOI
- Rezanka, T., Siristova, L., Sigler, K. (2009) Sterols and Triterpenoids with Antiviral Activity. Anti-Infective Agents in Medicinal Chemistry, 8(3), 193-210. DOI
- Chang, F.-R., Yen, C.-T., EI-Shazly, M., Lin, W.-H., Yen, M.-H., Lind, K.-H., Wu, Y.-C. (2012) Anti-Human Coronavirus (anti-HCoV) Triterpenoids from the Leaves of Euphorbia neriifolia. Natural Product Communications, 7(11), 1415-1417.
- Yang, J.-L., Ha, T.-K.-Q., Dhodary, B., Pyo, E., Nguyen, N.H., Cho, H., Kim, E., Oh, W.K. (2015) Oleanane Triterpenes from the Flowers of Camellia japonica Inhibit Porcine Epidemic Diarrhea Virus (PEDV) Replication. J Med Chem., 58(3), 1268-1280. DOI
- Yang, J.-L., Ha, T.K.Q., Oh, W.K. (2016) Discovery of inhibitory materials against PEDV corona virus from medicinal plants. Japanese Journal of Veterinary Research, 64(1), 53-63. DOI
- Ryu, Y.B., Park, S.-J., Kim, Y.M., Lee, J.-Y., Seo, W.D., Chang, J.S., Park, K.H., Rho, M.-C., Lee, W.S. (2010) SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorganic & Medicinal Chemistry Letters, 20(6), 1873-1876. DOI
- Tolkachev, O.N., Tolkachev, V.N., Sheichenko, O.P., Fateeva, T.V., Semenov, A.V., Abizov, E.A. (2018) Indole alkaloids and their analogues: biological activity study. Problems of biological, medical and pharmaceutical chemistry, (9), 3-14. DOI
- Lu, Y., Zhou, J., Hu, T., Zhang, Y., Su, P., Wang, J., Gao, W., Huang, L. (2018) A multifunctional oxidosqualene cyclase from Tripterygium regelii that produces both a- and bamyrin. RSC Adv., 8, 23516-23521. DOI
- Chen, C.-J., Michaelis, M., Hsu, H.-K., Tsai, C.-C., Yang, K.D., Wu, Y.-C., Cinatl, J., Doerrc, H.W. (2008) Toona sinensis Roem tender leaf extract inhibits SARScoronavirus replication. Journal of Ethnopharmacology, 120, 108-111. DOI