|
A New Field of Application of Saliva Tests for Prognostic Purpose: Focus on Lung Cancer
1Biochemistry Research Laboratory, Omsk State Pedagogical University, Key words: saliva; biochemical composition; lung cancer; overall survival; prognosis; adenocarcinoma; squamous cell carcinoma; neuroendocrine cancer DOI: 10.18097/BMCRM00133 INTRODUCTION
Lung cancer is the most common malignant neoplasm in the world population [1, 2]. Traditional tumor characteristics, such as differentiation, tumor invasion, lymph node metastasis, and TNM stage classification, are not the only parameters used for evaluation of disease prognosis [3-5]. For prognostic purposes, the following groups of biomarkers are considered: genetic (mutations, changes in gene copy number, mRNA expression), epigenetic (changes in the DNA methylation profile), proteomics (changes in the protein expression profile), and metabolic (changes in the spectrum of low molecular weight metabolites), synthesis profile and the level of miRNAs [6-9]. Currently, various hematological and biochemical markers common in routine clinical practice have been increasingly used for the prognostic prediction in lung cancer. For example, the ratio of albumin and fibrinogen levels [10], lactate dehydrogenase (LDH) activity [11, 12], the ratio of albumin and alkaline phosphatase activity [13], uric acid [14, 15], and selenium [16] are considered as prognostically important parameters. Parameters associated with the processes of hypoxia (osteopontin, carbonic anhydrase and LDH); inflammation (IL-6, IL-8, IL-10, C-reactive protein), and tumor markers (CEA, CYFRA 21-1) are considered as potential prognostic criteria [17-26]. Biomarkers associated with the immunological response (e.g. alpha-2-macroglobulin, serum IL-2 receptor, toll-like receptor 4, vascular endothelial growth factor [27]) are also considered in the context of lung cancer evaluation. Along with blood biomarkers, saliva biomarkers are becoming increasingly popular for diagnostics and prognosis of various oncological diseases [28-31]. Nevertheless, in the literature there are limited data on the study of the composition of saliva in lung cancer [32-35]. We have previously shown that saliva reflects metabolic changes that occur in lung cancer, and can be used both for diagnostic and prognostic purposes [36]. Prognostically unfavorable parameters include the level of imidazole compounds in saliva above 0.478 mmol/L and LDH activity below 545 U/L (HR = 4.17; 95% CI 1.36 - 12.51; р = 0.00000) [36]. Our results suggest that the use of biochemical markers of saliva can be used for prognostic purposes in lung cancer. In this paper, we consider the potential prognostic role of biochemical saliva parameters in dependence of the histological type of lung cancer, as well as the type of treatment. MATERIALS AND METHODS Participants The work was based on the results of examination and treatment of 425 patients cured at the Thoracic Department of the Clinical Oncology Center of Omsk during 2014-2017. At the time of inclusion in the study, patients did not undergo any treatment, including surgery, chemotherapy, or radiation. In each case, histological verification of the diagnosis was performed. The clinicopathological parameters such as age, gender, histologic subtype, lymphatic invasion, lymph node or distant metastasis and pathologic stage were evaluated. In all patients, lung cancer of various histological types was confirmed, including: 189 - adenocarcinoma (ADC), 135 - squamous cell cancer (SCC), 8 - mixed (ADC + SCC), 68 - neuroendocrine cancer and 25 - undifferentiated lung cancer (Table 1). The neuroendocrine cancer group included 16 patients with the diagnosis of typical and atypical carcinoid (low grade G1 + G2) and 52 patients with small cell and large cell lung cancer (high grade G3).
Radical surgery was performed in 230 (54.1%) patients, including 198 patients with lobectomy / bilobectomy, and 32 patients with pneumonectomy. The combined treatment was performed in 118 (27.8%) patients. The presence of metastases in the intrathoracic lymph nodes (N1, N2) was considered as the indication for combination treatment (besides the radical surgery). As the second stage of such treatment it included remote radiation therapy with daily dose splitting up to a total focal dose of 46 Gy. Radiation treatment as monotherapy was performed in 52 (12.2%) patients, chemotherapeutic treatment in 59 (13.9%) patients, and combined (radiation + chemotherapy) in 35 (8.2%) patients. Based on results of diagnostic tests, 49 (11.6%) patients did not receive any special treatment. Collection, Processing and Storage of Saliva Samples All participants had saliva sampling (5 mL) before treatment. Saliva samples were collected after overnight fast, between 8 and 10 a.m., after rinsing the oral cavity with water followed by saliva spit into sterile polypropylene tubes; the salivation rate (mL/min) was calculated as described in [37]. Saliva samples were centrifuged (10000 g for 10 min) (CLb-16, Russia) and biochemical analyses were performed immediately without storage and freezing. Biochemical Analysis of Saliva Samples The biochemical composition of the samples was established using the StatFax 3300 semi-automatic biochemical analyzer (Awareness Technology, USA) [38]. The pH, mineral composition (calcium, phosphorus, sodium, potassium, magnesium, chlorides, nitric oxide), the content of urea, total protein, albumin, uric acid, α-amino acids, imidazole compounds, seromucoids and sialic acids, the activity of enzymes (aminotransferases; alkaline phosphatase; LDH; gamma-glutamyl transpeptidase; α-amylase) were determined in all samples. The content of lipoperoxidation products (conjugated dienes, conjugated trienes, Schiff bases, malondialdehyde), the level of middle molecule toxins, and the activity of antioxidant enzymes (catalase, superoxide dismutase, peroxidase, total antioxidant activity) were as described in [35, 39]. Statistical Analysis The statistical analysis was performed by means of Statistica 13.3 EN (StatSoft, Tulsa, OK, USA). The total observation time was 6 years, the median observation time was 42 months. The overall survival (OS) of the patient was evaluated from the date of hospitalization to the date of the last observation (censored) or the date of death of the patient (complete). Survival data were obtained until December 2019. Evaluation of OS was performed using the Kaplan Mayer method with the presentation of survival curves and the calculation of the significance of differences in Log-rank. A univariate Cox proportional hazards regression analysis was initially variables carried out to investigate the relationships between salivary parameters and survival data. Finally, variables with p˂0.10 were chosen to formulate multivariate Cox proportional hazards regression models and determine the independent prognostic factors for OS. Hazard ratio (HR) was obtained with 95% confidence interval (CI). RESULTS Analysis of the Overall Survival of Lung Cancer Patients During the observation period, 297 people died. The median overall survival was 18.0 months; 63% of patients lived more than a year after the start of treatment, 31% of patients lived more than 3 years, and 20.5% of patients lived more than 5 years (Fig.1A).
Due to the heterogeneity of the study group, it was necessary to analyze the influence of individual factors on survival rates (Table 2). Although the age and gender of patients did not have statistically significant effects on survival rates a tendency towards a decrease in the overall survival rate with disease progression was clearly observed (Table 2). The comparison of the histological type of lung cancer revealed that the maximum survival rate was characteristic for adenocarcinoma (23.2 months); for squamous and neuroendocrine lung cancer with a high degree of malignancy, the risk was higher (Fig.1B). The lowest overall survival rate was typical for the group of patients with undifferentiated lung cancer (5.3 months). The survival rates of patients with neuroendocrine cancer of low malignancy G1 and G2 (typical and atypical carcinoids) should be considered separately. The median overall survival rate of these patients was 48.9 months (Table 2). It was shown that the overall survival of patients after radical surgery was 45.3%; this is significantly higher than the corresponding values for combined (24.3%) and palliative (0.78%) treatment (Table 2).
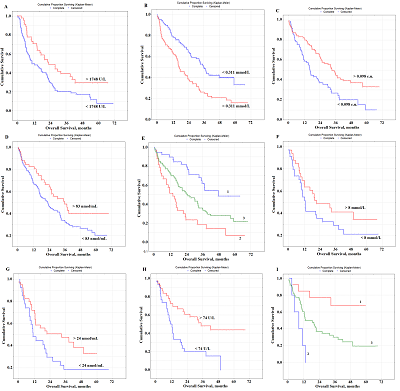
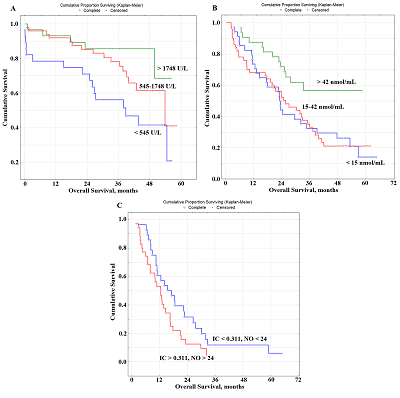
Prognostic Parameters Taking into Account the Histological Type of Lung Cancer At the next stage, prognostic parameters were determined taking into account the histological type of lung cancer. It was found that LDH activity was the only selected parameter prognostically important for squamous cell lung cancer (Fig. 2A). The level of saliva LDH below 1748 U/L before treatment (HR=2.89; 95% CI 1.28–6.46; p = 0.00330) was a prognostically unfavorable. The median overall survival with a favorable prognosis was two times higher than the corresponding value with an unfavorable prognosis (20.3 months vs 10.6 months).
In the case of lung adenocarcinoma, three parameters were selected as prognostic signs: the content of IC, seromucoids, and uric acid (Fig. 2B,C,D). The median concentrations of these indicators in the saliva of lung cancer patients were used as the threshold values: IC – 0.311 [0.197; 0.478] mmol/L, seromucoids – 0.098 [0.055; 0.154] c.u., uric acid – 83.33 [36.54; 166.67] nmol/mL (Supplementary Materials, Table S1). It was shown that the levels of IC˂0.311 mmol/L, seromucoids>0.098 c.u. and uric acid>83 nmol/l were prognostically favorable (Table 3, Fig.2B-D). The combination of these indicators allowed us to identify groups with favorable and unfavorable prognoses (Fig. 2E); for these groups the 1-year overall survival was 89.8% and 51.4%, and the 3-year overall survival was 61.7% and 18.8% and 5-year overall survival was 48.5% and 6.9%, respectively.
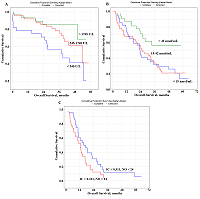
Several prognostically important parameters were also found for neuroendocrine lung tumors: urea concentration, nitric oxide (NO) and alkaline phosphatase (ALP) activity (Fig. 2F-H). The median concentrations of these parameters in the saliva of patients with lung cancer (urea – 8.00 [5.76; 11.86] mmol/L, NO – 24.0 [14.2; 42.3] nmol/mL, and alkaline phosphatase – 73.88 [49.98; 117.34] U/L) were used as threshold values (Supplementary Materials, Table S1). In this case, only the concentration of uric acid in saliva was an independent prognostic sign (Table 3). It was shown that a favorable prediction corresponded to the values of all parameters above the threshold level, while an unfavorable prediction was associated with the values below the threshold level (Table 3). It should be noted that in the group of patients with an unfavorable prognosis, all patients died by the end of the first year, while in the group with a favorable prognosis, 1-year overall survival was 84.3%, 3- and 5-year-survival was 68.2% (Fig. 2I) Prognostic Parameters Based on the Type of Treatment For both radical and combined treatments, as prognostically important parameters, only one of the saliva parameter was selected: LDH for radical treatment, NO for combined treatment (Fig. 3).
In both cases, to determine the prognosis, the concentration variation interval was divided into tertiles in accordance with the interquartile range: for LDH, 1133 [545.5; 1748.9] U/L, for NO - 24.0 [14.2; 42.3] nmol/mL (Table 3). It was shown that the value of LDH activity exceeding 1748 U/L for radical treatment (Fig. 3a) and the NO level exceeding 42 nmol/mL for combined treatment (Fig. 3B) were prognostically favorable. For palliative treatment, the levels of IС and NO were prognostically significant. In this case, the values of these indicators below the threshold level were prognostically favorable (Table 3). The combination of both indicators was more informative (Fig. 3c). So, in the case of a favorable prognosis, 1-, 3- and 5-year overall survival rates were 60.7%, 11.8%, and 6.2%, respectively. In the case of an unfavorable prognosis, the 1-year overall survival rate was 53.1%, while no patients survived for 3 years after treatment. DISCUSSION Biochemical saliva markers can be used for prognostic purposes in lung cancer. It should be noted that saliva biochemical markers have not been considered yet for prognostic purposes, not only for lung cancer, but also for other oncological diseases. Previously, we found the level of imidazole compounds in saliva and LDH activity was of high prognostic significance. This result is logical, because the metabolic changes accompanying lung cancer are characterized by a decrease in LDH activity, which corresponds to a decrease in the intensity of anaerobic and aerobic energy processes [40, 41]. It is also known that LDH activity can affect the malignant potential of a tumor by increasing proliferation, viability and invasive capacity of tumor cells, as well as by decreasing apoptosis [42, 43]. Our data showed that increased LDH activity in saliva was a prognostically favorable factor, whereas the opposite tendency was previously noted for blood plasma [11, 12, 44]. However, for most biochemical saliva markers, correlations with the composition of blood plasma were weak or even absent; therefore, the values of biochemical saliva markers should be considered as independent and criteria for norm and pathology should be developed for them [45]. The mean LDH activity in the group of lung cancer patients does not practically differ from the corresponding values of the control group (1133.0 U/L vs. 1127.5 U/L). However, with an increase in the tumor size, LDH activity decreased significantly (-12.1% from T1M0 to T2M0, -29.3% from T1M0 to T3-4M0). Similarly, with an increase in the degree of lymph node damage, LDH activity in saliva decreased (Supplementary Materials, Tables S3, S4). The prognostic value of the level of imidazole compounds has not been previously mentioned in the literature, while a decrease in the level of imidazole compounds in saliva is favorable. Imidazole compounds include the amino acid histidine and its metabolites (histamine, urocanilic acid, etc.), which are actively involved in malignant growth processes [46]. Tumor cells can secrete histamine, which generally affects the change in the metabolism of polyamines, and can lead to the formation of reactive oxygen species involved in carcinogenesis [47-49]. We have shown that with lung cancer, histamine levels are a parameter for monitoring the disease [50]. The level of imidazole compounds in saliva of lung cancer patient was higher than in the control group (0.311 mmol/L vs. 0.281 mmol/L); the maximum increase in this indicator corresponded to the T4M0 stage (+58.0%). The level of imidazole compounds increases with the increase in the degree of damage to the lymph node lesion (+17.4% for N1M0, +39.5% for N2M0, +77.9% for N3M0). Thus, a combination of increased LDH activity and a low content of imidazole compounds may be used to distinguish a group of patients with a favorable prognosis. In the case of a favorable prognosis the combination of both parameters corresponds to a median of overall survival of 22.4 months, whereas in the case of an unfavorable prognosis, this parameter is two times less (11.7 months). However, when the histological type of the tumor is taken into account, the prognostic nature of salivary LDH is preserved only for squamous cell lung cancer (Fig. 2A). We previously found that squamous cell carcinoma of the lung was characterized by a decrease in LDH activity (Supplementary Materials, Table S2) [40]. This suggests more pronounced effects of hypoxia in squamous cell carcinoma of the lung [51, 52]. In the case of favorable prognosis, the median overall survival was 20.3 months, while for squamous lung cancer this value was 15.2 months (HR=0.53; 95% CI 0.26-1.09; p – 0.05870). For lung adenocarcinoma, in addition to imidazole compounds, seromucoids and uric acid are selected as prognostic signs (Fig. 2B-E, Table 3). The level of seromucoids rises against lung cancer; however, as the disease progresses, seromucoid content decreases; this is the most pronounced for the common stages of the disease (Supplementary Materials, Table S3, S4) [50]. Uric acid is the end product of the metabolic cleavage of purine nucleotides. An increase in uric acid levels may result from the rapid catabolism of purine nucleic acids from cancer cells, and indicates a higher turnover of nucleic acids in cellular elements [53, 54]. Uric acid can be a mediator of free radical reactions, and act as an antioxidant [55]. It contributes to the total antioxidant ability of plasma [56]. There is evidence that uric acid is able to protect lipoproteins from oxidative stress [57]. This probably explains, why increased levels of seromucoids and uric acid are a prognostically favorable sign. An increase in the median overall survival with a favorable prognosis is also statistically significant as compared with the average values for lung adenocarcinoma (36.2 months vs. 23.2 months; HR=0.40; 95% CI 0.20–0.79; p – 0.00150). For the group with the unfavorable prognosis, statistically significant differences were also found (14.2 vs. 23.2 months; HR=3.15; 95% CI 1.16–8.41; p – 0.00097). Since the proportion of neuroendocrine tumors in the total sample size is small (16%), it is not surprising that the prognostically important parameters differ from those originally selected for the combined lung cancer group. These include urea, NO, and ALP (Table 3, Fig.2F-I). ALP activity was significantly higher in patients with neuroendocrine lung tumors than in the control group (Supplementary, Table S2). This is consistent with published data [58, 59]. Additionally, the difference in the ALP level between tumors of different degrees of malignancy is shown, namely: increased ALP activity for lung carcinoid tumors [60]. However, ALP activity decreases during disease progression. This is explained by the fact that the local stages of the disease without regional and distant metastases are mainly tumors of the G1 + G2 type, for which ALP activity is higher. The concentration of urea in saliva, like NO, decreases with the progression of the disease, the maximum values of these parameters are characteristic of the early stages of the disease (Supplementary Materials, Tables S3, S4). A decrease in the urea concentration with progression of lung cancer correlates with a decrease in the total protein concentration. Moreover, effective treatment and destruction of the tumor are accompanied by increased protein breakdown and so the level of urea will be higher. Good evidence exists that NO is a universal regulator of cell and tissue metabolism in tumor growth [61-63]. Importantly, NO effects at different disease stages are spatially-, temporally regulated and concentration-dependent [64]. High NO levels lead to nitrosative stress and activation of various stress-related proteins [65]. Probably, an increase in the NO level promotes the antitumor effect. Thus, for neuroendocrine tumors, an increase in all three parameters above a threshold value will be prognostically favorable. Comparison of the average values for the overall survival of lung cancer patients with and the neuroendocrine group with a favorable prognosis showed that the risk of dying in the group reduced (HR=0.25; 95% CI 0.07-0.88; p – 0.00006). Moreover, the medians of overall survival were 11.1 months and 33.5 months, respectively. This also confirms the good prospects for using these biochemical saliva markers to predict lung cancer. Depending on the type of treatment, the same parameters that were selected in other cases remain prognostically significant, however their combinations differed. For radical surgery, which is usually carried out at the early stages of the disease, salivary LDH activity is significant (Table 3, Fig. 3). For combined treatment, an elevated level of NO is prognostically favorable. It can be assumed that with an increase in the NO level, the content of active nitrosyl radicals increases; under conditions of radiation therapy this contribute to the compensation of its side effects. The manifestation of the antioxidant properties of NO can contribute to a better treatment effect and an increase in overall survival rates [66]. Moreover, the level of NO retains its prognostic value also with palliative chemo- and radiation therapy; however, it is not an independent prognostic sign. In this case the level of imidazole compounds, which statistically significantly increases at the advanced stages of the disease, is an additional parameter (Supplementary Materials, Tables S3, S4). These indicators can be used to predict the course of the disease and personalized patient’s care. Limitations of the study are associated with an insufficiently large group of patients. This seriously limits evaluation of the predictive power in subgroups of patients with different stages of lung cancer, histological types of cancer and treatments. It is also planned in the future to consider the prognostic value of isoforms of LDH and alkaline phosphatase enzymes in saliva in lung cancer. CONCLUSIONS For the first time, the possibility of using biochemical saliva parameters to determine the prognosis of lung cancer has been shown. The factors of favorable and unfavorable prognosis for lung cancer of various histological types, as well as depending on the type of treatment, which can be used in clinical practice to select the optimal tactics for treating patients, are established. FUNDING This work financed from the State Budget of the Omsk State Medical University and Omsk State Pedagogical University. COMPLIANCE WITH ETHICAL STANDARDS The study was carried out in accordance with the Helsinki Declaration (adopted in June 1964 in Helsinki, Finland and revised in October 2000 in Edinburgh, Scotland) and approved at a meeting of the Ethics Committee of the Omsk Regional Clinical Hospital "Clinical Oncology Center" on July 21, 2016 (Protocol No. 15). CONFLICT OF INTERESTS The authors declare that they have no conflict of interest. SUPPLEMENTARY Supplementary materials are available at http://dx.doi.org/10.18097/BMCRM00133 REFERENCES
|