Biosensor Selection of Small Compounds, Modulating a Complex Formation between Cytochrome P450s and Nadph-Dependent P450 Oxidoreductase
1Institute of Biomedical Chemistry", Pogodinskaya str. 10, bldg 8, Moscow, 119121Russia; *e-mail: evgeyablokov1988@mail.ru
2Institute of Bioorganic Chemistry of National Academy of Science of Belarus, Kuprevicha str. 5/2, Minsk, 220141, Republic of Belarus.
Keywords:affinity modulators; kinetics modulators; low molecular weight compounds; NADPH; cytochrome P450; NADPH-dependent cytochrome P450 oxidoreductase
DOI:10.18097/BMCRM00134
The study of the effect of low-molecular-weight compounds (substrates, endogenous metabolites, drugs and xenobiotics) on the kinetic and equilibrium parameters of functionally significant binary protein-protein interactions (PPIs) is of both fundamental and clinical importance. The surface plasmon resonance (SPR) is the method of the first choice for studying PPIs. Earlier, SPR analysis revealed the modulating effect of steroidal substrates on the affinity of interactions between steroidogenic microsomal cytochromes P450 (CYP) and their redox partner (cytochrome b5). In this work, we have shown the suitability of the experimental approach for assessing the selective effect of the cofactor NADPH on the interaction between cytochromes CYP3A4 or CYP2E1 with NADPH-dependent P450 oxidoreductase (CPR).
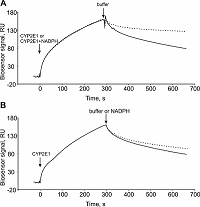
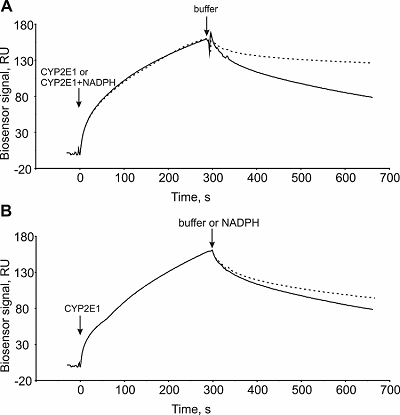
Experiments have shown that the CYP3A4/CPR complex is not modulated by NADPH, while the dissociation rate of the CYP2E1/CPR complex in the presence of NADPH significantly decreased: the koff values in the absence and presence of NADPH were (3.6 ± 0.2) • 10-3 s-1 and (3.8 ± 0.2) • 10-4 s-1, respectively. Thus, in the presence of NADPH, an increase in the affinity of CYP2E1/CPR complex formation by approximately one order of magnitude was observed, while NADPH did not affect the kon value of this complex. Co-injection of NADPH at the CYP2E1/CPR complex preformed in the absence of NADPH had minor influence on the koff values (<10%). This suggests a stabilizing role of NADPH for the CYP2E1/CPR complex formation. Thus, the use of our approach made it possible to assess the effect of the main electron supplier for the microsomal cytochrome P450 monooxygenase system on the kinetic rate constants of CYP/CPR complexes.
|
CLOSE

|
Table 1.
Association (kon) and dissociation (koff) rate constants and equilibrium dissociation constant (Kd) characterizing complex formation of cytochromes P450 with their redox partners in the absence/presence of the NADPH cofactor.
|
|
CLOSE

|
Table 2.
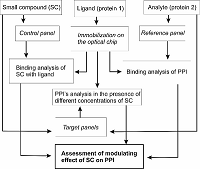
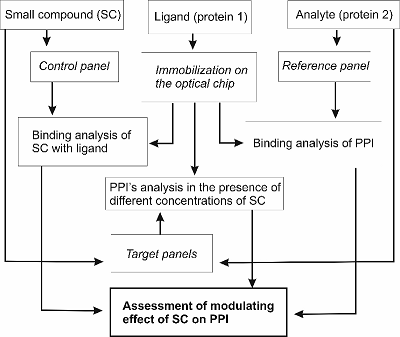
A typical experiment aimed at the detection of the modulating effect of a small compound (SC) on kinetics (affinity) of binary BBI.
|
FUNDING
This work was supported by the Russian Foundation for Basic Research (RFBR) (project № 18-04-00071).
REFERENCES
- Andrei, S. A., Sijbesma, E., Hann, M., Davis, J., O’Mahony, G., Perry, M. W. D., Karawajczyk, A., Eickhoff, J., Brunsveld, L., Doveston, R. G., Milroy, L.-G., Ottmann, C. (2017). Stabilization of protein-protein interactions in drug discovery. Expert Opinion on Drug Discovery, 12(9), 925–940. DOI
- Fischer, G., Rossmann, M., Hyvönen, M. (2015). Alternative modulation of protein-protein interactions by small molecules. Current Opinion in Biotechnology, 35, 78–85. DOI
- Cossar, P. J., Ma, C., Gordon, C. P., Ambrus, J. I., Lewis, P. J., McCluskey, A. (2017). Identification and validation of small molecule modulators of the NusB-NusE interaction. Bioorganic & Medicinal Chemistry Letters, 27(2), 162–167. DOI
- Ershov, P. V., Yablokov, Е. O., Florinskaya, A. V., Mezentsev, Y. V., Kaluzhskiy, L. А., Tumilovich, A. M., Gilep, А. А., Usanov, S. A., Ivanov, А. S. (2019). SPR-Based study of affinity of cytochrome P450s / redox partners interactions modulated by steroidal substrates. Journal of Steroid Biochemistry and Molecular Biology, 187, 124–129. DOI
- Ershov, P. V., Mezentsev, Y. V., Yablokov, E. O., Kaluzhsky, L. A., Florinskaya, A. V., Buneeva, O. A., Medvedev, A. E., Ivanov, A. S. (2018). Effect of Bioregulator Isatin on Protein–Protein Interactions Involving Isatin-Binding Proteins. Russian Journal of Bioorganic Chemistry, 44(2), 193–198. DOI
- Ershov, P. V., Yablokov, E. O., Mezentsev, Yu. V., Kalushskiy, L. A., Florinskaya, A. V., Veselovsky, A. V., Gnedenko, O. V., Gilep, A. A., Usanov, S. A., Medvedev, A. E., Ivanov, A. S. (2017). The effect of isatin on protein-protein interactions between cytochrome b5 and cytochromes P450. Biomeditsinskaya Khimiya, 63(2), 170–175. DOI
- Svirid, A. V., Ershov, P. V., Yablokov, E. O., Kaluzhskiy, L. A., Mezentsev, Y. V., Florinskaya, A. V., Sushko, T. A., Strushkevich, N. V., Gilep, A. A., Usanov, S. A., Medvedev, A. E., Ivanov, A. S. (2017). Direct Molecular Fishing of New Protein Partners for Human Thromboxane Synthase. Acta Naturae, 9(4), 92–100.
- Higashimoto, Y., Sakamoto, H., Hayashi, S., Sugishima, M., Fukuyama, K., Palmer, G., Noguchi, M. (2005). Involvement of NADPH in the interaction between heme oxygenase-1 and cytochrome P450 reductase. Journal of Biological Chemistry, 280(1), 729–737. DOI
- Esteves, F., Campelo, D., Gomes, B. C., Urban, P., Bozonnet, S., Lautier, T., Rueff, J., Truan, G., Kranendonk, M. (2020). The Role of the FMN-Domain of Human Cytochrome P450 Oxidoreductase in Its Promiscuous Interactions with Structurally Diverse Redox Partners. Frontiers in Pharmacology, 11, 299. DOI
- Bistolas, N., Wollenberger, U., Jung, C., Scheller, F. W. (2005). Cytochrome P450 biosensors-a review. Biosensors & Bioelectronics, 20(12), 2408–2423. DOI
- Iijima, M., Ohnuki, J., Sato, T., Sugishima, M., Takano, M. (2019). Coupling of Redox and Structural States in Cytochrome P450 Reductase Studied by Molecular Dynamics Simulation. Scientific Reports, 9(1), 9341. DOI
- Gutierrez, A., Munro, A. W., Grunau, A., Wolf, C. R., Scrutton, N. S., Roberts, G. C. K. (2003). Interflavin electron transfer in human cytochrome P450 reductase is enhanced by coenzyme binding. Relaxation kinetic studies with coenzyme analogues. European Journal of Biochemistry, 270(12), 2612–2621. DOI
- Xia, C., Hamdane, D., Shen, A. L., Choi, V., Kasper, C. B., Pearl, N. M., Zhang, H., Im, S.-C., Waskell, L., Kim, J.-J. P. (2011). Conformational changes of NADPH-cytochrome P450 oxidoreductase are essential for catalysis and cofactor binding. Journal of Biological Chemistry, 286(18), 16246–16260. DOI
- Kaluzhskiy, L. A., Ershov, P. V., Shkel, T. V., Gnedenko, O. V., Ivanchina, N. V., Strushkevich, N. V., Kicha, A. A., Grabovec, I. P., Gilep, A. A., Usanov, S. A., Stonik, V. A., Ivanov, A. S. (2018). Application of the SPR Biosensor in Drug Prototypes Discovery with Human Cytochrome P450(51) as an Example. Biomedical Chemistry: Research and Methods, 1(4), e00055. DOI
- Ershov, P., Mezentsev, Y., Gilep, A., Usanov, S., Buneeva, O., Medvedev, A., Ivanov, A. (2017). Isatin-induced increase in the affinity of human ferrochelatase and adrenodoxin reductase interaction. Protein Science, 26(12), 2458–2462. DOI