|
Optimization of Conditions for Human Bacterial Preparation for Biological Correction of Intestinal Microflora
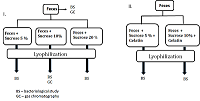
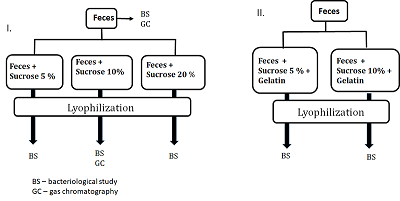
Federal Research and Clinical Center of Physical-Chemical Medicine of FMBA, Key words: fecal microbiota transplantation; lyophilized oral capsules; lyoprotectants; short-chain fatty acids DOI: 10.18097/BMCRM00151 INTRODUCTION Restoration of bacterial diversity in the gut by means of fecal microbiota transplantation (FMT) has become an effective method of treating and preventing recurrence of intestinal infection caused by a vancomycin-resistant form of Clostridium difficile [1]. Since 2013, the procedure for treatment of this condition has been officially approved by the U.S. Food & Drug Administration [2, 3]. The range of application of the method constantly expands. Today it is used as part of the complex therapy of inflammatory bowel diseases [4], correction of microbiota after antibiotic therapy [5], as well as an experimental technique in neurodegenerative disorders [6, 7]. The use of FMT leads to a decrease in the representation of pathogens with multiple resistance to antibiotics, which is extremely important against the background of increasing mortality due to infection with antibiotic-resistant pathogenic bacteria. Compared with many other medical procedures, FMT technique is very simple. However, its practical implementation is often complicated due to logistical barriers related to donor identification and verification, coordination of timing of material collection and preparation. These and other organizational barriers are overcome by the use of frozen feces [8, 9]. The main issue related to the use of frozen feces is the viability of microorganisms compared to the original material. In studies conducted on the use of FMT for the treatment of a vancomycin-resistant form of the Clostridium difficile, both freshly prepared and frozen feces did not differ in effectiveness, which was 81-100% [8, 9]. Another significant issue related to the use of frozen feces is the viability of microorganisms over time. Various studies report the use of frozen feces for 6 months of storage [10] and even 1 year [11] without loss of efficacy. Fecal preparations are delivered both through the lower gastrointestinal tract (enema, colonoscopy) and the upper gastrointestinal tract (endoscopy, oral capsules). Each method has its own advantages and disadvantages and is a choice of medical personnel. A common disadvantage of instrumental methods of introduction (endoscopy, colonoscopy) is their invasiveness associated with the risk of intestinal perforation and the use of anesthesia. Oral capsules filled with lyophilized feces are minimally invasive, convenient and more aesthetic, so in the absence of contraindications doctors and patients increasingly prefer this method of drug delivery [12, 13]. During lyophilization, microbial cells are exposed to stress factors such as low temperatures, water crystallization, osmotic stress, changes in solutions pH, and dehydration. To reduce the likelihood of cell damage during lyophilization, protective media, the main variable factor affecting the survival of bacteria during drying, are used [14]. The selection of protective media is carried out empirically. This is largely due to the fact that the mechanisms of bacterial cell damage during drying and storage of lyophilisate preparations have not been fully determined, as well as the mechanisms of protective action of lyoprotectors, the ideas about which are at the level of assumptions. Protective media should protect cells from damage during freezing, dehydration, storage, be easily dried, well soluble during rehydration, and have no toxicity. The range of effective lyo-protectors corresponding to the above properties is limited to carbohydrates, some amino acids, reduced milk, serum, gelatin and their combinations [15]. The most popular lyo-protectors are low-molecular-weight carbohydrates. Their protective properties in drying bacteria have been confirmed by numerous studies [16, 17]. Disaccharides are the most effective lyo-protectors [17], which are used in working concentrations of 5-20 % either individually or together with protectors of other chemical nature [15, 17]. According to the majority of works, the protective properties of disaccharides increase in the lactose-sucrose-trehalose series [18]. The aim of this work was to optimize the conditions of lyophilization of biomaterial for fecal transplantation, assess the viability of microorganisms, and compare the molar ratios of short-chain fatty acids in the obtained lyophilisates compared with the original fecal samples MATERIALS AND METHODS A volunteer donor's feces obtained after natural defecation served as the material for the study. A volunteer donor met the donor selection criteria for inclusion in the project «Establishment of a healthy donor fecal biobank at FRCC» approved by the FRCC Local Ethics Committee on September 6, 2016. In study phosphate-buffered saline (PBS, in tablets, «Biolot», Russia), sucrose («Sigma-Aldrich», USA), and food gelatin were used. Preparation of fecal samples for lyophilization In this work (a) sucrose and (b) a mixture of sucrose and food gelatin as lyoprotectants were studied. (a) A fresh donor fecal sample (40 g) was diluted in sterile 0.1 M phosphate buffered saline, pH 7.4. (The final volume ratio feces:PBS was 1:4). A household immersion blender with a 0.7-liter chopper volume (Philips, China) was used to obtain a homogeneous mass. Homogenization was performed for 1 min. The resulting suspension was filtered through a tea strainer (hole diameter 1-2 mm) and poured into 50 ml sterile plastic freezer jars («Sarstedt», Germany). The calculated amount of sucrose (2 g, 4 g, 8 g, respectively) was added to mixtures depending on the chosen percentage concentration (5%, 10%, 20%) and the final volume of the mixture (40 ml). The volume of mixture was brought to 40 ml with phosphate buffer. Each prepared sample (40 ml) was poured into 2 glass beakers for lyophilization and frozen at -80°C. (b) Preparation of fecal samples for lyophilization with a mixture of sucrose and gelatin was performed in same way. The feces were diluted in phosphate buffer containing 1% gelatin, prepared according to the manufacturer's instructions. Qualitative and quantitative bacteriological composition of the initial feces and lyophilized mixtures were studied according to the methodical recommendations «Bacteriological diagnostics of dysbiosis», approved by the Ministry of Health of Russia on April 14, 1997 and the methodical recommendations «Bacteriological diagnostics of diseases caused by Enterobacteriaceae», approved by the Ministry of Health of Russia in 2001 at the bacteriological laboratory of the Center. Gas chromatography determination of short-chain fatty acids (SCFAs) was performed on a Crystal 5000.2 gas chromatograph («Khromatek», Russia) with a flame ionization detector (FID) and a capillary chromatographic column of Carbowax cross-linked stationary liquid phase (length 30 m, internal diameter 0.32 mm, film thickness - 1.0 μm) according to the method developed earlier [19]. Statistical processing of the results was performed using Statistica 10.0 software package for Windows. The reliability of the results was assessed by Pearson's linear correlation criterion, values greater than 0.85 were considered significant. RESULTS AND DISCUSSION After analyzing the literature data on the use of protective media for lyophilization [16-18, 20, 21] for further practical work, sucrose was chosen as the most described in the scientific literature and available from the whole range of lyoprotectants. In addition, a mixture of sucrose and gelatin was used. The experimental design is shown in Figure 1. Bacteriological examination and gas chromatography analysis of the original fecal sample was performed, after which it was lyophilized with various selected sucrose concentrations of 5%, 10%, and 20%. Bacteriological and gas chromatography studies were also performed with the obtained lyophilisates. Before the bacteriological study, a visual assessment of the structure of the obtained lyophilisates was performed for their practical suitability for manual capsule preparation. Samples with sucrose content of 5% and 10% had an airy texture well crumbled into fine particles resembling "coffee powder" convenient for capsule preparation. The lyophilisates did not change their physical properties over time. The sample with a sucrose content of 20% turned out to be unsuitable for further work because it had no flowability, was poorly crushed and became hygroscopic with time. Lyophilisates containing sucrose-gelatin lyoprotectant mixture also were excluded from further work since after lyophilization they partially foamed forming a porous structure unsuitable for crushing into a homogeneous powder and further technological use.
To determine the microbial composition of the analyzed biomaterial (fresh feces, lyophilisates with sucrose content of 5% and 10%), a bacteriological study was performed. The qualitative composition of the microbiota population and quantitative representation of bacteria of the genus Bifidobacterium, Lactobacillus, Escherichia and the family Enterobacterales in total were evaluated during the study. Special attention was paid to the species related to normal intestinal microflora - Bifidobacteria and Lactobacilli [20]. It is known that normal intestinal microflora creates conditions for optimal digestion and absorption processes in the intestine, protects against pathogenic agents and stimulates the body's immune response [20]. According to the data obtained in the lyophilized samples, the quantitative representation of Bifidobacteria and Lactobacilli did not change compared with the original material (fresh feces) (Table 1). The representation of Enterobacteriaceae, Enterococci, and Escherichia coli - decreased significantly (by 2 orders of magnitude on average). This is probably due to the different sensitivity of microorganisms to temperature changes (freezing-thawing). According to literature data, Gram-negative bacteria are often more sensitive to freezing than Gram-positive ones, which is explained by peculiarities of the cell wall structure [21].
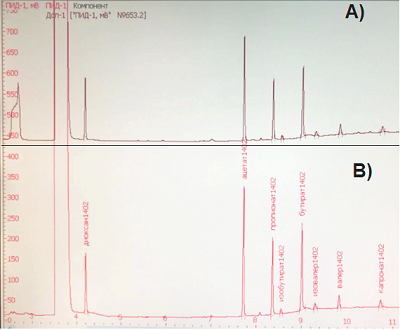
The peculiarity of this sample is the increased growth of bacteria of the Proteus genus. These microorganisms are representatives of normal, conditionally pathogenic microflora of human intestine. However, to some extent, their presence was reflected in the final results. Bacteria of the Proteus genus are characterized by very active motility and are capable of suppressing the growth of other microorganisms characterized by slower growth. This may make it difficult to identify other species of microorganisms. This observation applies mainly to E. coli, whose representation in the lyophilized samples was somewhat reduced. In general, analysis of the bacterial composition of donor stool samples (fresh and lyophilized) suggests a comparable microbial landscape of the studied preparations. Despite the fact that the bacterial composition in lyophilisates containing 5% and 10% sucrose was virtually identical, for further work and subsequent technological use lyophilisate with sucrose content of 10%, was chosen since this concentration of lyoprotectant according to literature data is more traditional for lyophilization of bacteria [15, 17]. Reproducibility of the results (the "survival rate" of bacteria in lyophilized fecal samples) was evaluated based on 6 experimental repeats. Pearson linear correlation coefficient between the series was r=0.853 The therapeutic effect of FMT is polyvalent and is associated not only with microflora replacement, but also with enrichment with “healthy” metabolites effect. These metabolites, namely short-chain fatty acids are produced by intestinal microflora. The molar ratio of major SCFAs (acetate, propionate and butyrate) in the large intestine and feces of healthy individuals is relatively constant and averages 60:20:20 [22], which allows their use for objective evaluation of the functional state of intestinal microbiota. The concentrations and molar ratios of SCFAs in the original fecal sample and the studied lyophylizates were analyzed by gas chromatography and were identical to each other (Fig. 2). The molar ratios of the major SCFAs in the test sample were 64:19:17.
COMPLIANCE WITH ETHICAL STANDARDS All procedures performed in human research comply with the ethical standards of the Institutional and/or National committee on research ethics and the 1964 Declaration of Helsinki and its subsequent amendments or comparable standards of ethics. The informed voluntary consent was obtained from the donor included in the study. FUNDING This study was performed within the framework of the State Assignment of the Federal Scientific and Clinical Center for Physico-Chemical Medicine CONFLICT OF INTEREST The authors declare that they have no conflict of interest. REFERENCES
|