Influence of Adding cRGD Peptide to Phospholipide Nanoparticles with Doxorubicin Included on Apoptosis in Glioblastoma Cells in Vitro
Institute of Biomedical Chemistry, Pogodinskaya str., 10, Moscow 119121, Russia; *e-mail: kostryukova87@gmail.com
Keywords: glioblastoma; phospholipid nanoparticles; cRGD; integrin αvβ3; doxorubicin; apoptosis
DOI:10.18097/BMCRM00204
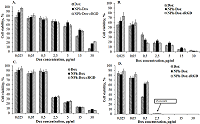
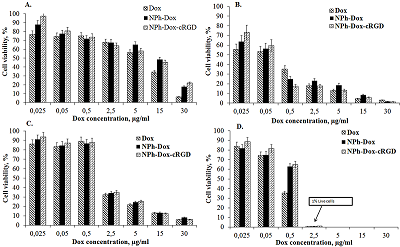
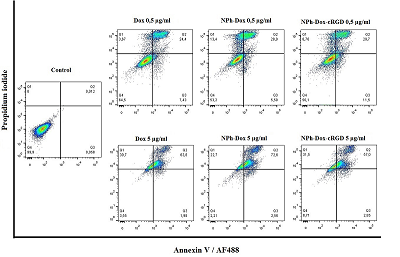
One of the methods of treating glioblastoma after surgery is chemotherapy; the drugs used in this case, due to their nonspecific distribution, lead to a number of complications. One way to overcome this drawback is to supply drugs with delivery systems with targeted molecules. This approach allows increasing the accumulation of therapeutic agents directly at the lesion site, minimizing side effects. This work is a continuation of the study of the mechanism of action of the previously obtained phospholipid composition of doxorubicin with a targeted cRGD peptide (NPh-Dox-cRGD). This peptide is capable of selectively interacting with integrin αvβ3, a receptor expressed on the surface of a number of tumor cells, including glioblastoma. The work assessed the cytotoxic effect of the NPh-Dox-cRGD composition in comparison with the free substance (Dox) and embedded in phospholipid nanoparticles without a targeted ligand (NPh-Dox). It was shown that after 24 h of incubation of U-87 MG cells with substances at the maximum concentration of Dox (30 μg/ml), the percentage of viability cells was 6% for Dox, 21% for NPh-Dox-cRGD, and 21% for NPh-Dox – 17%, i.e. When Dox was incorporated into phospholipid NPs, its cytotoxic effect was observed to a lesser extent. No statistically significant differences were noted in the control line HeLa. Assessment of tumor cell death using flow cytometry indicated that most of the cells died via apoptosis. When incubated with a composition containing a targeting peptide, NPh-Dox-cRGD, at a concentration (Dox) of 0.5 μg/ml, the percentage of cells susceptible to late apoptosis was 29.7%, for the free form - 24.4%. An assessment of cells susceptible to early apoptosis (Dox concentration 0.5 µg/ml) showed that the percentage of these cells for the sample with the peptide was higher and amounted to 11.4%.
|
CLOSE

|
Table 1.
Calculated value IC50 µg/ml obtained on the U-87 MG cell line after 24 h of incubation.
|
FUNDING
The work was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021-2030) ((№ 122030100170-5).
REFERENCES
- Liu, D., Dai, X., Ye, L., Wang, H., Qian, H., Cheng, H., Wang, X. (2023) Nanotechnology meets glioblastoma multiforme: Emerging therapeutic strategies. Wiley interdisciplinary reviews. Nanomedicine and Nanobiotechnology, 15(1), e1838. DOI
- Schaff, L. R., Mellinghoff, I. K. (2023) Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA, 329(7), 574–587. DOI
- Yang, J., Li, Y., Zhang, T., & Zhang, X. (2016) Development of bioactive materials for glioblastoma therapy. Bioactive Materials, 1(1), 29–38. DOI
- Meng, L., Chu, X., Xing, H., Liu, X., Xin, X., Chen, L., Jin, M., Guan, Y., Huang, W., & Gao, Z. (2019). Improving glioblastoma therapeutic outcomes via doxorubicin-loaded nanomicelles modified with borneol. International Journal of Pharmaceutics, 567, 118485. DOI
- Sági, J. C., Egyed, B., Kelemen, A., Kutszegi, N., Hegyi, M., Gézsi, A., Herlitschke, M. A., Rzepiel, A., Fodor, L. E., Ottóffy, G., Kovács, G. T., Erdélyi, D. J., Szalai, C., Semsei, Á. F. (2018) Possible roles of genetic variations in chemotherapy related cardiotoxicity in pediatric acute lymphoblastic leukemia and osteosarcoma. BMC Cancer, 18(1), 704. DOI
- Maksimenko, O., Malinovskaya, J., Shipulo, E., Osipova, N., Razzhivina, V., Arantseva, D., Yarovaya, O., Mostovaya, U., Khalansky, A., Fedoseeva, V., Alekseeva, A., Vanchugova, L., Gorshkova, M., Kovalenko, E., Balabanyan, V., Melnikov, P., Baklaushev, V., Chekhonin, V., Kreuter, J., Gelperina, S. (2019) Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. International Journal of Pharmaceutics, 572, 118733. DOI
- Shafei, A., El-Bakly, W., Sobhy, A., Wagdy, O., Reda, A., Aboelenin, O., Marzouk, A., El Habak, K., Mostafa, R., Ali, M. A., Ellithy, M. (2017) A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomedicine & Pharmacotherapy, 95, 1209–1218. DOI
- Weekes, C. D., Vose, J. M., Lynch, J. C., Weisenburger, D. D., Bierman, P. J., Greiner, T., Bociek, G., Enke, C., Bast, M., Chan, W. C., Armitage, J. O., Nebraska Lymphoma Study Group (2002) Hodgkin's disease in the elderly: improved treatment outcome with a doxorubicin-containing regimen. Journal of clinical oncology, 20(4), 1087–1093. DOI
- Fekih, L., Boussoffara, L., Fenniche, S., Abdelghaffar, H., Akrout, I., Ayadi, A., Megdiche, M. L. (2011) Sarcome primitif rare de la paroi thoracique: le synovialosarcome [Rare primary chest wall sarcoma: the synovialosarcoma]. Revue des Maladies Respiratoires, 28(5), 681–685. DOI
- Aljarrah, K., Mhaidat, N. M., Al-Akhras, M. A., Aldaher, A. N., Albiss, B., Aledealat, K., & Alsheyab, F. M. (2012) Magnetic nanoparticles sensitize MCF-7 breast cancer cells to doxorubicin-induced apoptosis. World Journal of Surgical Oncology, 10, 62. DOI
- Tacar, O., Sriamornsak, P., Dass, C. R. (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. The Journal of Pharmacy and Pharmacology, 65(2), 157–170. DOI
- Wang, K., Zhang, X., Liu, Y., Liu, C., Jiang, B., & Jiang, Y. (2014) Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials, 35(30), 8735–8747. DOI
- Thorpe, P. E., Chaplin, D. J., & Blakey, D. C. (2003) The first international conference on vascular targeting: meeting overview. Cancer Research, 63(5), 1144–1147
- Wang, K., Shen, R., Meng, T., Hu, F., Yuan, H. (2022) Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer. Molecules (Basel, Switzerland), 27(9), 2981. DOI
- Torchilin V. P. (2010) Passive and active drug targeting: drug delivery to tumors as an example. Handbook of Experimental Pharmacology, 197, 3–53. DOI
- Cheng, Y., Ji, Y. (2019) RGD-modified polymer and liposome nanovehicles: Recent research progress for drug delivery in cancer therapeutics. European Journal of Pharmaceutical Sciences, 128, 8–17. DOI
- Pisano, M., Dе Paola, I., Nieddu, V., Sassu, I., Cossu, S., Galleri, G., Del Gatto, A., Budroni, M., Cossu, A., Saviano, M., Palmieri, G., Zaccaro, L., Rozzo, C. (2013) In vitro activity of the αvβ3 integrin antagonist RGDechi-hCit on malignant melanoma cells. Anticancer Research, 33(3), 871–879
- Godugu. K., Sudha, T., Davis, P.J., Mousa, S.A. (2021) Nano Diaminopropane tetrac and integrin αvβ3 expression in different cancer types: Anti-cancer efficacy and Safety. Cancer Treat. Res. Commun., 28, 100395. DOI
- Li, H., Peng, W., Zhen, Z., Zhang, W., Liao, S., Wu, X., Wang, L., Xuan, A., Gao, Y., & Xu, J. (2023). Integrin αvβ3 and EGFR dual-targeted [64Cu]Cu-NOTA-RGD-GE11 heterodimer for PET imaging in pancreatic cancer mouse model. Nuclear Medicine And biology, 124-125, 108364. DOI
- Chen, W., Zou, Y., Zhong, Z., & Haag, R. (2017) Cyclo(RGD)-Decorated Reduction-Responsive Nanogels Mediate Targeted Chemotherapy of Integrin Overexpressing Human Glioblastoma In Vivo. Small, 13(6). DOI
- Miura, Y., Takenaka, T., Toh, K., Wu, S., Nishihara, H., Kano, M.R., Ino, Y., Nomoto, T., Matsumoto, Y., Koyama, H. (2013) Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano, 7(10), 8583–8592. DOI
- Zhan, C., Gu, B., Xie, C., Li, J., Liu, Y., Lu, W. (2010) Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. Journal of controlled release: official journal of the Controlled Release Society, 143(1), 136–142. DOI
- Zhan, C., Wei, X., Qian, J., Feng, L., Zhu, J., Lu, W. (2012) Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo. Journal of Controlled Release, 160(3), 630–636. DOI
- Waite, C. L., Roth, C. M. (2009) PAMAM-RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant glioma. Bioconjugate Chemistry, 20(10), 1908–1916. DOI
- Kostryukova, L.V., Tereshkina, Yu.A., Tikhonova, E.G., Sanzhakov, M.A., Bobrova, D.V., Khudoklinova, Yu.Yu. (2022) Study of the efficiency of cellular accumulation of doxorubicin supplied with a targeted delivery system based on phospholipid nanoparticles with integrin-directed peptide. Biomeditsinskaya Khimiya, 68(6), 437-443. DOI
- IC50 Calculator. Retrieved September 2, 2023 from https://www.aatbio.com
- Wang, F., Li Y., Shen, Y., Wang, A., Wang, S., Xie, T. (2013) The functions and applications of RGD in tumor therapy and tissue engineering. International Journal of Molecular Sciences, 14(7), 13447-13462. DOI
- TerBush, A. A., Hafkamp, F., Lee, H. J., & Coscoy, L. (2018). A Kaposi's Sarcoma-Associated Herpesvirus Infection Mechanism Is Independent of Integrins α3β1, αVβ3, and αVβ5. Journal of Virology, 92(17), e00803-18. DOI
- Xiao, Y., Hong, H., Javadi, A., Engle, J. W., Xu, W., Yang, Y., Zhang, Y., Barnhart, T. E., Cai, W., Gong, S. (2012). Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging. Biomaterials, 33(11), 3071–3082. DOI
- Xiao, Y., Hong, H., Javadi, A., Engle, J.W., Xu, W., Yang, Y., Zhang, Y., Barnhart, T.E., Cai, W., Gong, S. (2012) Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging. Biomaterials, 33(11), 3071-3082. DOI
- Wang, Y., Hun, W., Ding, B., Chen, D., Cheng, L. (2020) cRGD mediated redox and pH dual responsive poly(amidoamine) dendrimer-poly(ethylene glycol) conjugates for efficiently intracellular antitumor drug delivery. Colloids Surf. B. Biointerfaces, 194, 111195. DOI
- Kciuk, M., Gielecińska, A., Mujwar, S., Kołat, D., Kałuzińska-Kołat, Ż., Celik, I., Kontek, R. (2023). Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells, 12(4), 659. DOI
- Zhu, L., Lin, M. (2021) The Synthesis of Nano-Doxorubicin and its Anticancer Effect. Anti-cancer agents in medicinal chemistry, 21(18), 2466–2477. DOI
- van Tellingen, O., Yetkin-Arik, B., de Gooijer, M.C., Wesseling, P., Wurdinger, T., de Vries, H.E. (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy, 19, 1-12. DOI