β1-Adrenergic Receptor Solubilized in the Form of Nanodiscs: Screening of Various Amphipatic Polymers
National Medical Research Center of Cardiology named after Academician E.I.Chazov , 15a Academician Chazov str., Moscow, 121552 Russia; *e-mail: tanya.vlasik@gmail.com
Keywords:autoantibodies to β1-adrenergic receptor; nanodiscs; amphipatic polymers
DOI:10.18097/BMCRM00206
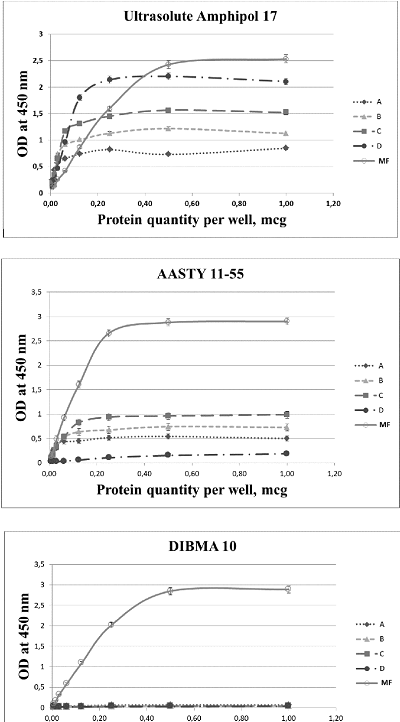
The development of a reliable and easily used diagnostic test for measuring autoantibodies to β1-adrenergic receptor (β1ADR Ab) in patient blood is an unmet clinical need. The enzyme-linked immunosorbent assay (ELISA) is considered as the most appropriate method for this task. In ELISA, the use of peptides corresponding to various fragments of amino acid sequence of β1ADR as antigens leads to inadequate results as β1ADR Ab appear to recognize conformationally dependent epitopes that are generated during the formation of unique tertiary structure of the receptor. Isolation of β1ADR preserving the native conformation and functional characteristics is a quite challenging task. A promising approach to address this task is the use of amphipatic polymers capable of forming nanodiscs, which permits to successfully solubilize membrane proteins. Here, we describe the results of testing 17 various amphipatic polymers with the aim to obtain the preparations of solubilized β1ADR that can be used as antigens in ELISA. The best relative solubilization value (RSV) were demonstrated by UltrasoluteTM Amphipol 17 (87%) and 18 (62%), as well as by AASTY 11-45 (76%), 11-50 (77%) and 6-50 (78.5%).
|
CLOSE

|
Table 1.
Relative solubilization value (RSV) at various polymer concentrations. Ultr Amph – UltrasoluteTM Amphipole.
|
|
CLOSE

|
Table 2.
Maximum RSV as function of molecular weight (MW) and structural features of polymers. S:MA – styrene:maleic acid ratio. S:MALEI – styrene:maleimide ratio. DIB:MA – diisobutylene:maleic acid ratio. AA:STY – acrylic acid:styrene ratio. X:Y – ratio of hydrophobic to hydrophylic units in polymer. Ultr Amphipole – UltrasoluteTM Amphipole.
|
FUNDING
This work is financed by the Ministry of Health of the Russian Federation (state assignment on the subject No. 152 “The development of the enzyme-linked immunosorbent assay for measuring autoantibodies to β1-adrenergic receptor in cardiac patients with a purified recombinant β1-adrenergic receptor in native conformation as an antigen”, Ref. No. NIOKTR 122020400212-0) and “Mona Ltd.” (Moscow, OGRN 5087746251896).
Supplementary materials are available at http://dx.doi.org/10.18097/BMCRM00206
REFERENCES
- Sterin-Borda, L., Cossio, P.M., Gimeno, M.F., Gimeno, A.L., Diez, C., Laguens, R.P. , Meckert, P.C., Arana, R.M.( 1976) Effect of chagasic sera on the rat isolated atrial preparation: immunological, morphological and function aspects. Cardiovasc. Res., 10(6), 613–622. DOI
- Magnusson, Y., Wallukat, G., Waagstein, F., Hjalmarson, A., Hoebeke, J. (1994) Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta1-adrenoceptor with positive chronotropic effect. Circulation, 89(6), 2760–2767. DOI
- Jahns, R., Boivin, V., Siegmund, C., Inselmann, G., Lohse, M.J., Boege, F. (1999) Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation, 99(5), 649–654. DOI
- Liu, J., Wang, Y., Chen, M., Zhao, W., Wang, X., Wang, H., Zhang, Z., Zhang, J., Xu, L., Chen, J., Yang, X., Zhang, L. (2014) The correlation between peripartum cardiomyopathy and autoantibodies against cardiovascular receptors. PLoS One, 9(1), e86770. DOI
- Magnusson, Y., Marullo, S., Hoyer, S., Waagstein, F., Andersson, B., Vahlne, A., Guillet, J.G., Strosberg, A.D., Hjalmarson, A., Hoebeke, J. (1990) Mapping of a functional autoimmune epitope on the beta1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J. Clin. Inves.t, 86(5), 1658–1663. DOI
- Wallukat, G., Wollenberger, A., Morwinski, R., Pitschner, H.F. (1995) Anti-beta1- adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J. Mol. Cell Cardiol., 27(1), 397–406. DOI
- Peclo, M.M., Lipatova, L.N., Gerasimova, E.I. (2020) Autoantibodies to β1-adrenergic receptor: pathogenetic role, mechanisms of action and methods of determination. Kardiol. Vestnik, 3, 20-25. DOI
- Felix, S.B., Staudt, A., Landsberger, M., Grosse, Y., Stangl, V., Spielhagen, T., Wallukat, G., Wernecke, K.D., Baumann, G., Stangl, K. (2002) Removal of cardiodepressant antibodies in dilated cardiomyopathy by immunoadsorption. J. Am. Coll. Cardiol., 39(4), 646–652. DOI
- Ronspeck, W., Brinckmann, R., Egner, R., Gebauer, F., Winkler, D., Jekow, P., Wallukat, G., Müller, J., Kunze, R. (2003) Peptide based adsorbers for therapeutic immunoadsorption. Ther Apher. Dial., 7(1), 91–97. DOI
- Wallukat, G., Muller, J., Hetzer, R. (2002) Specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N. Engl. J. Med., 347(22), 1806-1806. DOI
- Störk, S., Boivin, V., Horf, R., Hein, L., Lohse, M.J., Angermann, C.E., Jahns, R. (2006) Stimulating autoantibodies directed against the cardiac ß1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am. Heart J, 152(4), 697–704. DOI
- Iwata, M., Yoshikawa, T., Baba, A., Anzai, T., Mitamura, H., Ogawa, S. (2001) Autoantibodies against the second extracellular loop of beta(1)-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol, 37(2), 418–424. DOI
- Pei, J., Li, N., Chen, J., Li, X., Zhang, Y., Wang, Z., Zhang, P., Cao, K., Pu, J. (2012) The predictive values of beta1-adrenergic and M2 muscarinic receptor autoantibodies for sudden cardiac death in patients with chronic heart failure. Eur. J. Heart Fail, 14(8), 887–894. DOI
- Wenzel, K., Schulze-Rothe, S., Haberland, A., Müller, J., Wallukat, G., Davideit, H. (2017) Performance and in-house validation of a bioassay for the determination of beta1- autoantibodies found in patients with cardiomyopathy. Heliyon, 3 (7), e00362. DOI
- Gupalo, E.M., Stukalova, O.V., Rogova, M.M., Mironova, N.A., Malkina, T.A., Sharf, T.V., Efremov, E.E., Gerasimova, V.V., Bakalov, S.A., Ternovoy, S.R., Golitsyn, S.P. (2014) Relationship of focal fibrosis according to magnetic resonance tomography, autoantibodies to cardiac membrane receptors and ventricular arrhythmias in patients with dilated cardiomyopathy. Kardiologya (12), 29-36.
- Matsui, S., Fu, M.L., Shimizu, M., Fukuoka, T., Teraoka, K., Takekoshi, N., Murakami, E., Hjalmarson, A. (1995) Dilated cardiomyopathy defines serum autoantibodies against G-protein coupled cardiovascular receptors. Autoimmunity; 21(2), 85–88. DOI
- Bornholz, B., Benninghaus, T., Reinke, Y., Felix, S.B., Roggenbuck, D., Jahns-Boivin, V., Jahns, R., Boege, F. (2015) A standardised FACS assay based on native, receptor transfected cells for the clinical diagnosis and monitoring of β1-adrenergic receptor autoantibodies in human heart disease. Clin. Chem. Lab. Med., 54(4), 1-9. DOI
- Bornholz, B., Hanzen, B.,Reinke, Y., Felix, S.B., Jahns, R., Schimke, I., Wallukat, G., Boege, F. (2016) Detection of DCM-associated β1-adrenergic receptor autoantibodies requires functional readouts or native human β1-receptors as targets. Int. J. Cardiol; 202, 728–730. DOI
- Krishnarjuna, B., Ramamoorthy A. (2022) Detergent-free isolation of membrane proteins and strategies to study them in a near-native membrane environment. Biomolecules, 12(8), 1076. DOI
- Sligar, S.G., Denisov, I.G. (2021) Nanodiscs: a toolkit for membrane protein science. Protein Sci., 30(2), 297–315. DOI
- Orekhov, P.S., Bozdaganyan, M.E., Voskoboynikova, N., Mulkidjanian, A.Y., Karlova, M.G., Yudenko, A., Remeeva, A., Ryzhykau, Y.L., Gushchin, I., Gordeliy, V.I., Sokolova, O.S., Steinhoff, H.J, Kirpichnikov, M.P., Shaitan K.V. (2022) Mechanisms of formation, structure, and dynamics of lipoprotein discs stabilized by amphiphilic copolymers: a comprehensive review. Nanomaterials, 12(3), 361. DOI
- Rues, R.B., Dötsch, V., Bernhard, F. (2016) Co-translational formation and pharmacological characterization of beta1-adrenergic receptor/nanodisc complexes with different lipid environments. Biochim. Biophys. Acta. 1858(6), 1306-1316. DOI
- Shevelev, A.Y., Kashirina, N.M., Kuznetsova, T.B., Sharf, T.V., Mamochkina, E.N., Agapova, O.Y., Gurskaya, T.K., Lipatova, L.N., Peklo, M.M., Rutkevich, P.N., Yanushevskaya, E.V., Rybalkin, I.N., Skoblov, Y.S., Efremov, E.E., Vlasik, T.N., Zykov, K.A. (2015) Cell line expressing recombinant β1-adrenergic receptor for the agonistic autoantibodies detection by a competitive enzyme-linked immunosorbent assay. Vestnik Biotechnol., 11(4), 5–14.
- Afanas’eva, O.I., Klesareva, E.A., Efremov, E.E., Sidorova, M.V., Bespalova, Zh.D., Levashov, P.A., Ezhov, M.V., Adamova, I.Yu., Pokrovsky, S.N. (2013) An immunoenzyme method based on chimeric molecule and oligopeptide fragments for determining the autoantibodies to β1-adrenergic receptor in patients with dilated cardiomyopathy. Zh. Klin. Lab. Diagn., (4), 24-27.
- Smirnova, I.A., Sjöstrand, D., Li, F., Björck, M., Schäfer, J., Östbye, H., Högbom, M., von Ballmoos, C., Lander, G.C., Ädelroth, P., Brzezinski P. (2016) Isolation of yeast complex IV in native lipid nanodiscs. Biochim. Biophys. Acta (BBA)-Biomembr. 1858(12), 2984–2992. DOI
- Szundi, I.; Pitch, S.G.; Chen, E.; Farrens, D.L.; Kliger, D.S. (2021) Styrene-maleic acid copolymer effects on the function of the GPCR rhodopsin in lipid nanoparticles. Biophys. J., 120(20), 4337–4348. DOI
- Mueller, S., Kubicek, J., Merino, F., Hanisch, P., Maertens, B., Lackmann, J.-W. (2023) The bigger picture: global analysis of solubilization performance of classical detergents versus new synthetic polymers utilizing shotgun proteomics. bioRxiv preprint. DOI
- Marconnet, A., Michon, B., Le Bon, C., Giusti, F., Tribet, C., Zoonens, M. (2020). Solubilization and stabilization of membrane proteins by cycloalkane-modified amphiphilic polymers. Biomacromolecules, 21(8), 3459–3467. DOI
- Sun, R., Mak, S., Haschemi, J., Horn P, Luppa, P.B. (2019) Nanodiscs incorporating native β1 adrenergic receptor as a novel approach for the detection of pathological autoantibodies in patients with dilated cardiomyopathy. J. Appl. Lab. Med. 4(3):391-403. DOI
- Kock, Z., Ermel, U., Martin, J., Morgner, N., Frangakis, A.S., Dotsch, V., Hilger, D., Bernhard, F. (2022) Biochemical Characterization of cell-free synthesized human β1 adrenergic receptor cotranslationally inserted into nanodiscs. J. Molecular. Biol., 434 (16), 167687. DOI
- Harwood,,C.R., Sykes, D.A., Hoare, B.L., Heydenreich, F.M., Uddin, R., Poyner, D.R., Briddon, S.J., Veprintsev, D.B. (2021) Functional solubilization of the β2-adrenoceptor using diisobutylene maleic acid. iScience, 24(12),103362. DOI
- Tedesco, D., Maj, M., Malarczyk, P., Cingolani, A., Zaffagnini, M., Wnorowski, A., Czapinski, J., Benelli, T., Mazzoni, R., Bartolini, M., Jozwiak, K. (2021) Application of the SMALP technology to the isolation of GPCRs from low-yielding cell lines. BBA – Biomembranes, 1863(9), 183641. DOI
- Wheatley, M., Charlton, J., Jamshad, M., Routledge, S.J., Bailey, S., La-Borde, P.J., Azam, M.T., Logan, R.T., Bill, R.M., Dafforn, T.R., Poyner D.R. (2016) GPCR–styrene maleic acid lipid particles (GPCR–SMALPs): their nature and potential. Biochem. Soc. Trans., 44(2), 619–623, DOI