Peroxynitrite Modification as a Way to Improve the Cytocompatibility of Sodium Alginate-Based Hydrogels
Ryazan State Medical University named after academician I.P. Pavlov,
9 Visokovoltnaya str., Ryazan, 390026 Russia; *e-mail: AlexanderZakharov2019@yandex.ru
Keywords:sodium alginate; gelatin; peroxynitrite; hydrogel; fibroblasts; cytocompatibility
DOI:10.18097/BMCRM00207
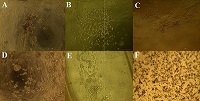
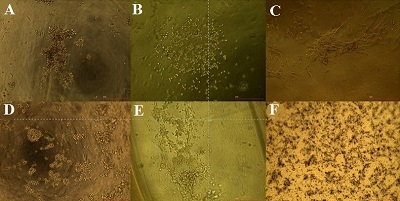
Sodium alginate is one of the most frequently used materials in biomedicine. However, alginate-based scaffolds have extremely low adhesive properties and have to be improved. The authors have proposed combined hydrogels of gelatin and sodium alginate which have been modified by peroxynitrite in heterophasic conditions using ethanol. It has been determined that thus modified sodium alginate contains an increased level of carbonyl, carboxyl and nitro groups. Authors have developed chemically and chemical-enzymatically cross-linked hydrogels with better adhesive properties and absence of cytotoxicity. Moreover, sodium alginate modification has a positive impact on cell morphology in comparison with control group of non-adhesive alginate-gelatin hydrogels. It allows the further improvement and application of the biomaterial which have been developed by the authors for bioengineering scaffold production and 3D culturing.

|
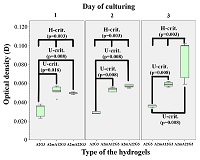
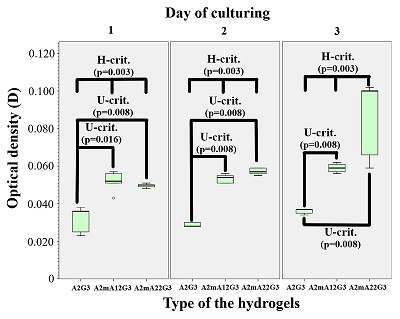
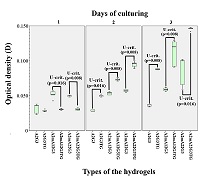
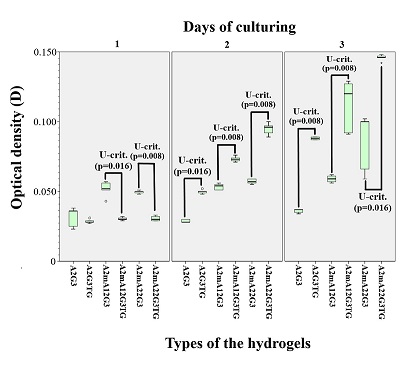
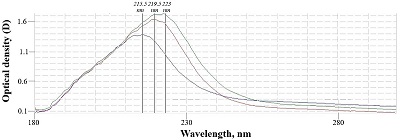
Figure 1.
Spectrophotometry of sodium alginates.
Blue indicates intact sample, red and green indicate the first and second modified samples, respectively.
|
|
CLOSE

|
Table 1.
Characterization of the obtained samples of modified sodium alginates
|
FUNDING
The study had no additional sources of funding.
REFERENCES
- Zakharov, A.S., Kalinin, R.E., Suchkov, I.A., Korotkova, N.V., Kovalev, S.A., Mzhavanadze, N.D. (2022) Modern possibilities of bioengineering in the creation of vascular grafts. Russian Journal of Thoracic and Cardiovascular Surgery, 3(64), 265–272. DOI
- Matveeva, V.G., Khanova, M.Yu., Glushkova, T.V., Antonova, L.V. (2021) Influence of different concentrations of fibrinogen on the properties of a fibrin matrix for vascular tissue engineering. I.P. Pavlov Russian Medical Biological Herald, 29(1), 21-34. DOI
- Buev, D.O., Emelin, A.M., Yakovlev, I.A., Deev, R.V. Cultivation of myoblasts and myosatellitocytes in vitro. Science of the young (Eruditio Juvenium), 8(1), 86-97. DOI
- Xing, Y., Qing, X., Xia, H., Hao, S., Zhu, H., He, Y., Mao, H., Gu, Z. (2021) Injectable Hydrogel Based on Modified Gelatin and Sodium Alginate for Soft-Tissue Adhesive. Front. Chem., 9, 744099. DOI
- Tarabah, F. (2015) Good manufacturing practice (GMP) for biomaterials and medical devices in the EU and the USA. Regulatory Affairs for Biomaterials and Medical Devices, 2015, 115–143. DOI
- Abdulghani. S., Mitchell. G.R. (2019) Biomaterials for in situ tissue regeneration: a review. Biomolecules, 9(11), 750. DOI
- Rastogi. P., Kandasubramanian. B. (2019) Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication, 11(4), 042001. DOI
- Łabowska. M.B., Cierluk. K., Jankowska. A.M., Kulbacka. J., Detyna. J., Michalak. I. (2021) A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials (Basel), 14(4), 858. DOI
- Putri. A., Picchioni. F., Harjanto. S., Chalid. M. (2021) Alginate modification and lectin-conjugation approach to synthesize the mucoadhesive matrix. Applied Sciences, 11, 11818. DOI
- Kong, X., Chen, L., Li, B., Quan, C., Wu, J. (2021) Applications of oxidized alginate in regenerative medicine. Journal of Materials Chemistry B, 9(12), 2785–2801. DOI
- Rajalekshmi, R., Kaladevi Shaji, A., Joseph, R., Bhatt, A. (2021) Scaffold for liver tissue engineering: Exploring the potential of fibrin incorporated alginate dialdehyde-gelatin hydrogel. Int. J. Biol. Macromol., 166, 999-1008. DOI