Rifampicin inhibits TLR4 and IL1β gene expression and enhances SH-SY5Y cell viability after prolonged ethanol exposure in an in vitro experiment
1Institute of Experimental Medicine, 12 Acad. Pavlova str., St. Petersburg, 197376 Russia
2Kirov Military Medical Academy, St. Petersburg, Russia
3North-Western State Medical University named after I.I. Mechnikov, St. Petersburg, Russia
4Saint-Petersburg National Research University of Information Technologies, Mechanics and Optics, St. Petersburg, Russia
5Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia
Keywords:peptide retention time; isoelectric point; post-translational modifications; web service
DOI:10.18097/BMCRM00208
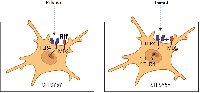
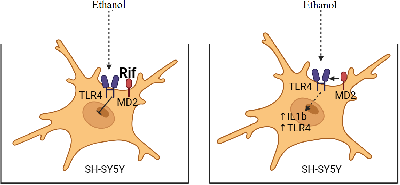
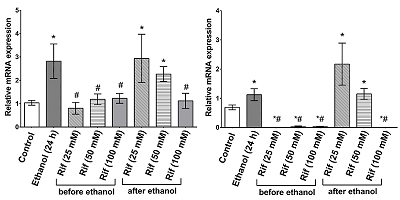
Prolonged alcohol exposure activates TLR4-signaling pathways in the brain, which is responsible for the development of neuroinflammation. There is interest in pharmacologic correction of these mechanisms. The antibiotic rifampicin (Rif) has established itself as a potential neuroprotectant in studies on its use to correct various pathologic conditions of the nervous system associated with the development of neuroinflammatory events. We performed a study on human neuroblastoma cell culture SH-SY5Y. Prolonged incubation of cells in ethanol (24 h, 100 mM) of SH-SY5Y induces activation of the innate immune system genes Tlr4 and Il1β. Pre-addition of rifampicin (25-100 mM) prior to incubation of cells in ethanol solution inhibited Tlr4 and Il1β gene expression, whereas addition of rifampicin after incubation of cells in ethanol dose-dependently reduced the increased expression of Tlr4 and Il1β genes, with the most significant effect at a concentration of 100 mM. In addition, the use of rifampicin increased cell survival in culture. Thus, the results of our experiment showed that Rif is able to eliminate the increased expression of inflammation genes caused by prolonged alcohol exposure and to increase the survival rate of long-term incubated cells in ethanol solution.
|
Figure 1.
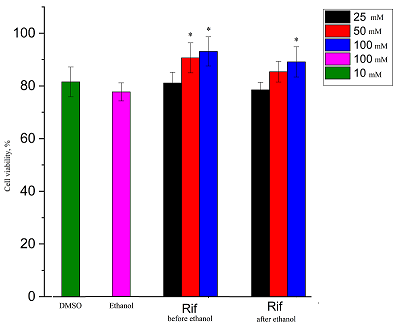
Viability of SH-SY5Y culture after 24 h incubation in ethanol solution (100 mM) and after 2 h incubation with DMSO and Rif (*p≤0.05 vs. ethanol).
|
|
Figure 2.
Relative level of TLR4 (left) and IL1β (right) mRNA content
(*p≤0.05 vs. control, #p≤0.05 vs. ethanol).
|
|
CLOSE

|
Table 1.
Sequence of primers.
|
FUNDING
The work was carried out within the framework of the State Task of the Ministry of Education and Science of Russia (2022-2025) “Search for molecular targets for pharmacological effects in addictive and neuroendocrine disorders and the creation of new pharmacologically active substances acting on CNS receptors”, code FGWG-2022-0004.
REFERENCES
- Airapetov, M.I., Eresko, S.O., Lebedev, A.A., Bychkov, E.R., Shabanov, P.D. (2020) The role of toll-like receptors in the neuroimmunology of alcoholism. Biomeditsinskaya Khimiya, 66(3), 208-215. DOI
- Crews, F.T., Zou, J., Qin, L. (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain, Behavior, and Immunity, 25(Suppl.1), 4-12. DOI
- Crews, F.T., Vetreno, R.P. (2014) Neuroimmune basis of alcoholic brain damage. Int. Rev. Neurobiol., 118, 315-357. DOI
- Crews, F.T., Vetreno, R.P. (2016) Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl), 233(9), 1543-57. DOI
- Coleman, L.G.J., Crews, F.T. (2018) Innate immune signaling and alcohol use disorders. Handb. Exp. Pharmacol., 248, 369-396. DOI
- Bi, W., Zhu, L., Wang, C. (2011) Rifampicin inhibits microglial inflammation and improves neuron survival against inflammation. Brain. Res., 1395, 12-20. DOI
- Yurtsever, İ., Üstündağ, Ü.V., Ünal, İ., Ateş, P.S., Emekli-Alturfan, E. (2022) Rifampicin decreases neuroinflammation to maintain mitochondrial function and calcium homeostasis in rotenone-treated zebrafish. Drug Chem. Toxicol., 45(4), 1544-1551. DOI
- Zahednasab, H., Firouzi M., Kaboudanian-Ardestani, S., Mojallal-Tabatabaei, Z., Karampour, S., Keyvani, H. (2019) The protective effect of rifampicin on behavioral deficits, biochemical, and neuropathological changes in a cuprizone model of demyelination. Cytokine, 113, 417-426. DOI
- Airapetov, M.I., Eresko, S.O., Ignatova, P.D., et al. (2023) The effect of rifampicin on expression of the toll-like receptor system genes in the forebrain cortex of rats prenatally exposed to alcohol. Biomeditsinskaya Khimiya, 69(4), 228-234. DOI
- Airapetov, M.I., Eresko, S.O., Skabelkin, D.A., et al. (2022) The effect of rifampicin on the system of toll-like receptors in the nucleus accumbens of the brain of long-term alcoholized rats during alcohol withdrawal. Biomeditsinskaya Khimiya, 68(4), 279-287. DOI
- Wang, X., Grace, P.M., Pham, M.N., Cheng, K., Strand, K.A., Smith, C., Li, J., Watkins, L.R., Yin, H. (2013) Rifampin inhibits toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J., 27(7), 2713-2722. DOI
- Bi, W., Cheng, X., Zeng, Z., Zhou, R., Luo, R., Zhang, J., Zhu, L. (2021) Rifampicin ameliorates lipopolysaccharide-induced cognitive and motor impairments via inhibition of the TLR4/MyD88/NF-κB signaling pathway in mice. Neurol. Res., 43(5), 358-371. DOI
- Kumar, P., Nagarajan, A., Uchil, P.D. (2018) Analysis of cell viability by the alamar blue assay. Cold Spring Harb. Protoc., 2018(6), 095489. DOI
- Livak, K.J., Schmittgen,T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods., 25(4), 402-408. DOI:10.1006/meth.2001.1262
- Yulug, B., Hanoglu, L., Kilic, E., Schabitz, W.R. (2014) RIFAMPICIN: an antibiotic with brain protective function. Brain Res. Bull., 107, 37-42. DOI
- Umeda, T., Uekado, R., Shigemori, K., Eguchi, H., Tomiyama, T. (2022) Nasal rifampicin halts the progression of tauopathy by inhibiting tau oligomer propagation in alzheimer brain extract-injected mice. Biomedicines, 10(2), 297. DOI
- Umeda, T., Hatanaka, Y., Sakai, A., Tomiyama, T. (2021) Nasal rifampicin improves cognition in a mouse model of dementia with lewy bodies by reducing α-synuclein oligomers. Int. J. Mol. Sci., 22(16), 8453. DOI
- Acuña, L., Hamadat, S., Corbalán, N.S. (2019) Rifampicin and its derivative rifampicin quinone reduce microglial inflammatory responses and neurodegeneration induced in vitro by α-synuclein fibrillary aggregates. Cells, 8(8), 776. DOI
- Getachew, B., Csoka, A.B., Tizabi, Y. (2022) Dihydromyricetin protects against ethanol-induced toxicity in SH-SY5Y cell line: role of GABAA receptor. Neurotox. Res., 40(3), 892-899. DOI
- Wernicke, C., Hellmann, J., Finckh, U., Rommelspacher, H. (2010) Chronic ethanol exposure changes dopamine D2 receptor splicing during retinoic acid-induced differentiation of human SH-SY5Y cells. Pharmacol. Rep., 62(4), 649-663. DOI
- Lawrimore, C.J., Coleman, L.G., Zou, J., Crews, F.T. (2019) Ethanol induction of innate immune signals across bv2 microglia and SH-SY5Y neuroblastoma involves induction of IL-4 and IL-13. Brain. Sci, 9(9), 228. DOI