The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Synthesis of Water-Soluble Phthalocyanines and Modification of their Peripheral Fragments as a Method of Directed Changes in Lipophilic-Hydrophilic Properties
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: ikalashn@ipac.ac.ru
Key words: water-soluble phthalocyanines; photosensitizer; peripheral substituents; template condensation
DOI: 10.18097/BMCRM00024
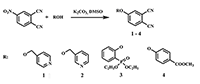
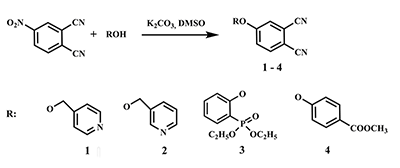
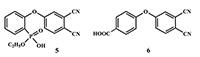
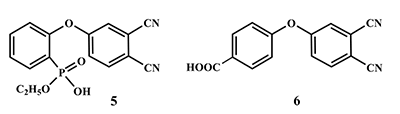
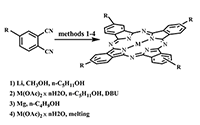
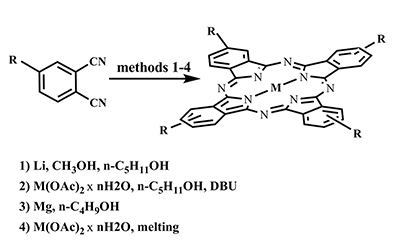
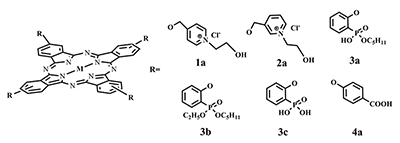
This article brief by describes results of studies devoted to synthesis of water-soluble phthalocyanines derivatives, which are carried out by the Complexing Agents Chemistry group of IPAC RAS. Metal-free ligands and metallophthalocyanines, containing peripheral substituents with fragments of pyridine, oxybenzoic and hydroxyphenylphosphonic acids are potential photosensitizers for photodynamic therapy of cancer diseases. Methods for the modification of peripheral substituents have been developed that make it possible to synthesize phthalocyanine ligands and complexes soluble in organic solvents, or soluble in aqueous media and also possess amphiphilic properties.
|
CLOSE

|
Table 1.
Lipophilic-hydrophilic properties of phthalocyanines.
|
ACKNOWLEDGEMENTS
This work was implemented within the framework of State task 2018 (No 0090-2017-0024 и 0081-2014-0015) and partial financial support by the Program for Basic Research No 34 and by the Russian Foundation for Basic Research grant no 18-03-00743).
REFERENCES
- Quirk B. J.,Brandal G., Donlon S.,Vera J. C., MangT. S., Foy A. B., Lew S. M., Girotti A. W., Jogal S., LaViolette P. S., Connelly J. M., Whelan H. T. (2015). Photodynamic therapy (PDT) for malignant brain tumors – Where do we stand? Photodiagnosis and Photodynamic Therapy, 12(3), 530-544. DOI
- Mehraban N., Freeman H. S. (2015). Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials, 8(7), 4421-4456. DOI
- Deda D. K., Araki K. (2015). Nanotechnology, Light and Chemical Action: an Effective Combination to Kill Cancer Cells. Journal of the Brazilian Chemical Society, 26(12), 2448-2470. DOI
- Li H., Jensen T. J., Fronczek F. R., Vicente M. G.(2008). Syntheses and properties of a series of cationic water-soluble phthalocyanines. Journal of Medicinal Chemistry. DOI
- Makhseed S., Machacek M., Alfadly W., Tuhl A., Vinodh M., Simunek T., Novakova V., Kubat P., Rudolf E., Zimcik P. (2013). Water-soluble non-aggregating zinc phthalocyanine and in vitro studies for photodynamic therapy. Chem. Commun., 49(95), 11149-11151. DOI
- Saydan N., Durmuş M., Dizge M. G., Yaman H., Gürek A. G., Antunes E., Nyokong T., Ahsen V., (2009). Water soluble phthalocyanines mediated photodynamic effect on mesothelioma cells. Journal of Porphyrins Phthalocyanines, 13(6), 681-690. DOI
- Güzel E., Koca A., Koçak M. B. (2017). Anionic water-soluble sulfonated phthalocyanines: microwave-assisted synthesis, aggregation behaviours, electrochemical and in-situ spectroelectrochemical characterisation. Supramolecular Chemistry, 29(7), 536-546. DOI
- Dumoulin F., Durmus M., Ahsen V., Nyokong T. (2010) Synthetic pathways to water-soluble phthalocyanines and close analogs. Coordination Chemistry Reviews. 254(23-24). 2792-2847. DOI
- Hanack M. (2015). Glycosylated Metal Phthalocyanines. Molecules 20(11), 20173-20185. DOI
- Hofman J.-W., van Zeeland F., Turker S., Talsma H., Lambrechts S. A. G., Sakharov D. V., Hennink W. E., van Nostrum C. F. (2007). Peripheral and Axial Substitution of Phthalocyanines with Solketal Groups: Synthesis and In Vitro Evaluation for Photodynamic Therapy. J. Med. Chem., 50(7), 1485–1494. DOI
- Chernonosov A. A.,.Ermilov E. A, Röder B., Solovyova L. I., Fedorova O. S. (2014), Effect of Some Substituents Increasing the Solubility of Zn(II) and Al(III) Phthalocyanines on Their Photophysical Properties. Bioinorganic Chemistry and Applications, Article ID 952632. DOI
- Kalashnikova I.P., Baulin V.E., Tsivadze A.Y. (2013). Synthesis and spectral characteristics of water-soluble pyridine-containing phthalocyanines of cation type. Russian Journal of General Chemistry. 83(10). 1910-1918. DOI
- Kalashnikova I.P., Baulin D.V., Baulin V.E., Tsivadze A.Yu. (2018.) Modification of phosphoryl substituents of phthalocyanines as a method of directed changes in lipophilic-hydrophilic properties. Russian Journal of General Chemistry. in press.
- Yurtseven H., Kaya M., Altındal A., Şener M. K. (2014) Synthesis, thermal, and electrical properties of stilbene-bridged polymeric zinc phthalocyanine, Designed Monomers and Polymers, 17(1), 58-68. DOI
- Ramos A. A., Nascimento F. B., de Souza T. F. M., Omori A.T., Manieri T.M., Cerchiaro G., Ribeiro A. O. (2015) Photochemical and Photophysical Properties of Phthalocyanines Modified with Optically Active Alcohols, Molecules 20(8), 13575-13590. DOI
- Hajri A., Touaiti S., Jamoussi B. (2013), Preparation of Organic Zn-Phthalocyanine-Based Semiconducting Materials and Their Optical and Electrochemical Characterization, Advances in OptoElectronics, Article ID 321563. DOI
- Maizlish, V.E., Martynyuk, T.A. Shaposhnikov, G.P. (2014). Preparation and properties of copper tetra-4-[(4′-carboxy)phenylamino]phthalocyanine. Russian Journal of General Chemistry, 84(1). 131-136. DOI