METAL-ORGANIC FRAMEWORK STRUCTURES IN MODERN RESEARCH: MEDICINE, DIAGNOSTICS, ECOLOGY
1Federal budgetary institution of science “State Research Center for Virology and Biotechnology “Vector” of Rospotrebnadzor, Koltsovo, Novosibirsk Region, 630559, Russia, e-mail: tumanov@vector.nsc.ru
1State University Dubna, University Str. 19, Dubna, Moscow region, 141980, Russia
Keywords:biosensors; MOC synthesis; MOC/COF hybrid structures; metal-organic frameworks; nucleic acids; bioimaging; fluorescence detection; fluorescence quencher
DOI:10.18097/BMCRM00270
The review describe modern technological developments of means of detection of viruses and toxins by using new nanomaterials based on frame structures. Synthesis and functionalization of metal-organic compounds of a frame structure (MOCs) and covalent organic frameworks (COF) are considered as well as the latest achievements in biomedical felds, including the delivery of drugs, nucleic acids, proteins and dyes for cancer therapy, bioimaging, antimicrobial drugs, biosensors and biocatalysis. Special attention is paid to new trends and promising areas in the development of biomedical materials based on MOC/COF. Data on the application of new biotechnological products based on simeconductor nanocrystals (quantum dots, QD) and their composites as part of MOCs in solving the problems of modern disease diagnostics that play a strategic role in the development of nanotechnology, biotechnology and nanomedicine are presented. Issues related to the recognition of biomolecules using hybrid MOC/COF structures are discussed. The use of QD nanocomposites with other carbon-based, grapheme-based or MOC-based nanomaterials resulted in the development of new systems for bioimaging, drug delivery, optogenetics and theranostics. Undoubtedly, the rapidly accumulating data on the behavior of QD/MOC in analytical systems in vitro will increase knowledge for the advancement of QD nanotechnology in research in vivo and clinical application.

|
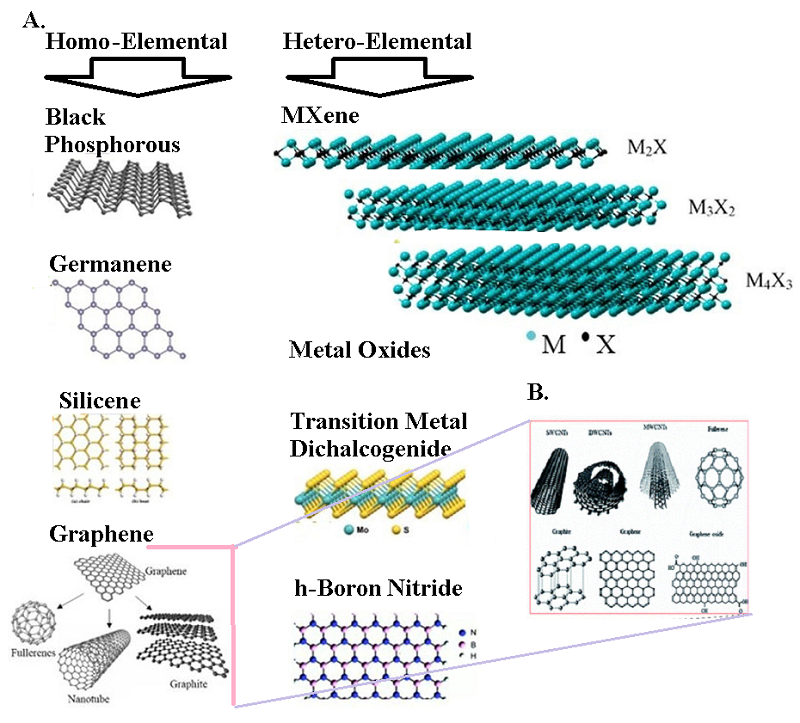
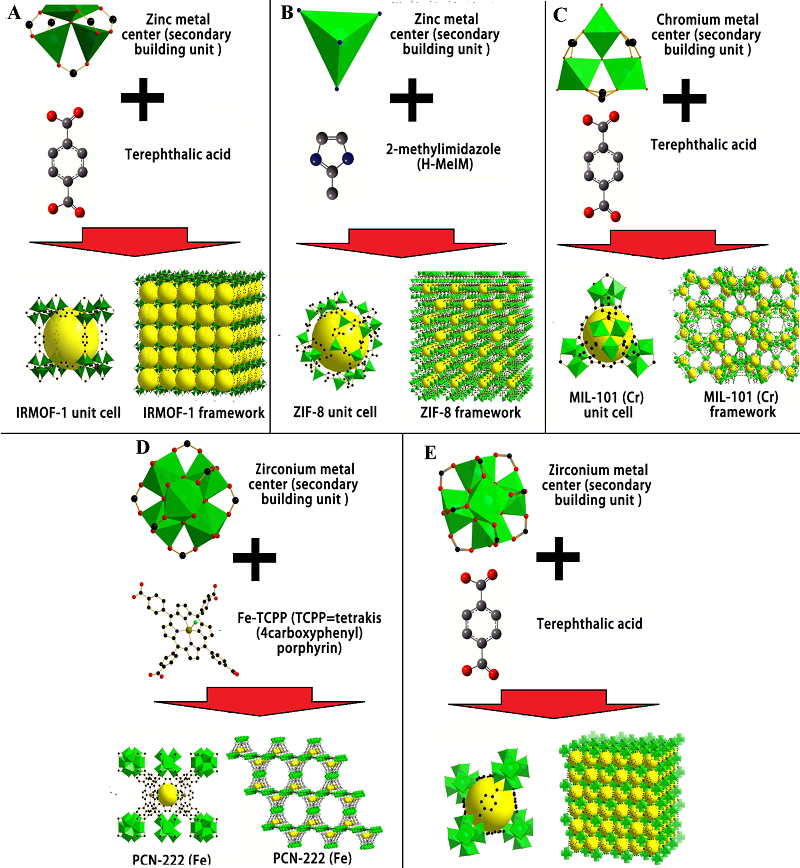
Figure 1.
Representative MOF types (adapted from [20]). Schematic illustrations of metal centers (secondary building units), organic linkers, unit cells, and frameworks of IRMOF-1 (a), ZIF-8 (b), MIL-101(Cr) (c), PCN-222(Fe) (d), and UiO-66 (e).
|

|
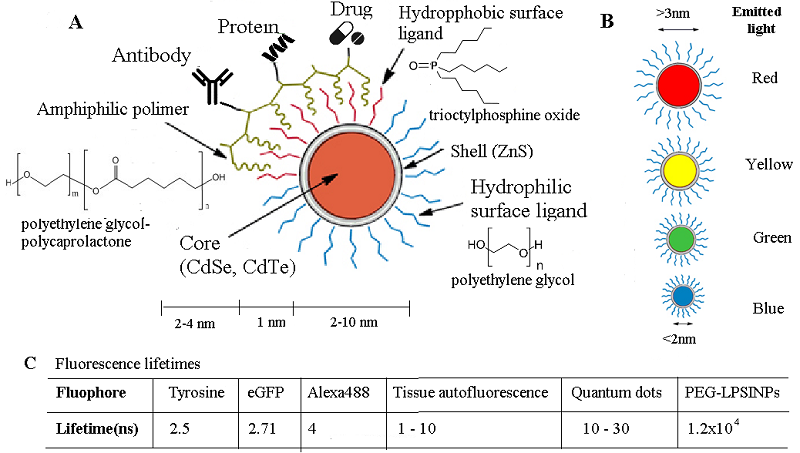
Figure 2.
The anatomy of quantum dots (adapted from [82]). (A) QDs contain a semiconducting core-shell. The surface can be coated with hydrophilic, hydrophobic, and amphiphilic ligands (common coating molecules are shown) which can be further linked with proteins, drugs, antibodies, and other compounds. (B) The emission spectra of QDs can be tuned by adjusting the size. (C) Fluorescence lifetimes of QD in comparison with other flurophores.
|

|
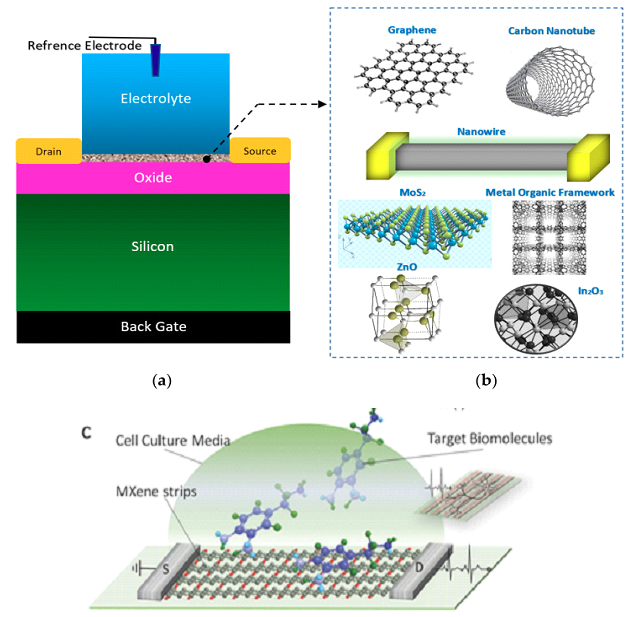
Figure 4.
The general form of electrolyte and back gated structures of FETs, which have been used for the sensing elements in Chem/BioFET: This nanomaterial-based structure has been widely used for cell, DNA, enzymatic reaction and chemical sensing; A - sensor structure; B - the most frequent nanomaterials used as sensing element and main conductive channel [103] (CC BY-SA 4.0). C - Schematic of a biosensor based on MXene FETs (adapted from [104]).
|

|
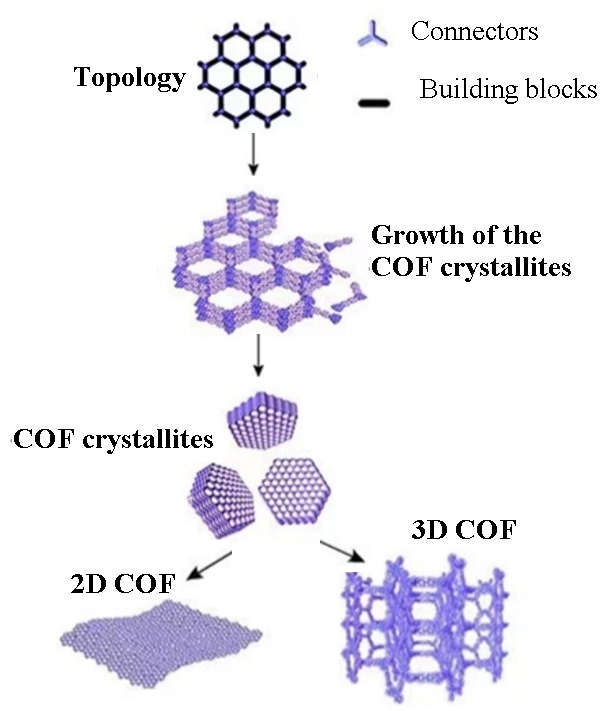
Figure 5.
The topology and growth of the COFs (adapted from [137]).
|

|
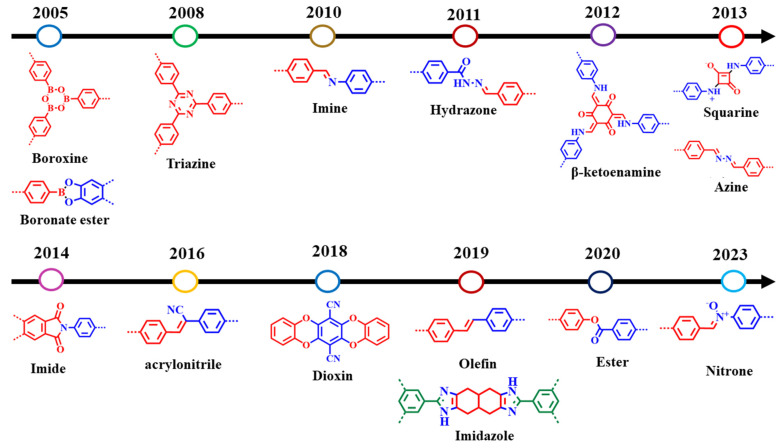
Figure 6.
Timeline of various dynamic linkages for COF formation [134] (CC BY-SA 4.0).
|

|
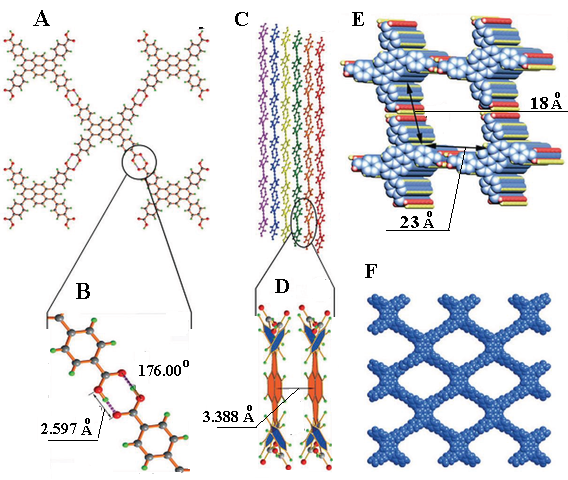
Figure 7.
Crystal structure of PFC-1. A - View of the structure and the connection of adjacent building blocks. B - Hydrogen-bond length and angle. C - The stacking of 2D layers. D - Face-to-face π–π interactions. e) One-dimensional (1D) channels. f) Representation of the porous framework (adapted from [198]).
|

|
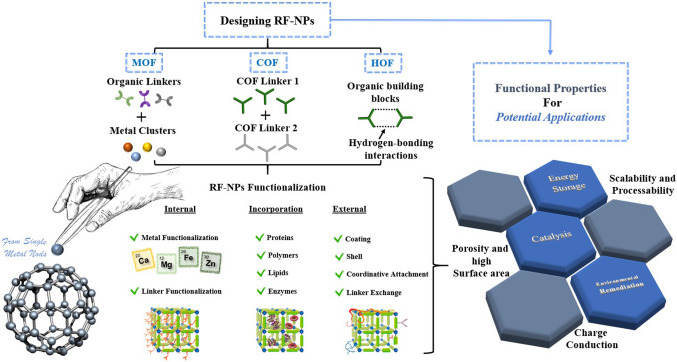
Figure 8.
Overview of functionalization pathways for RF-NPs. Internal and external surface modification as well as guest incorporation for various intrinsic and extrinsic properties enabling diverse applications [203] (CC BY-SA 4.0).
|

|
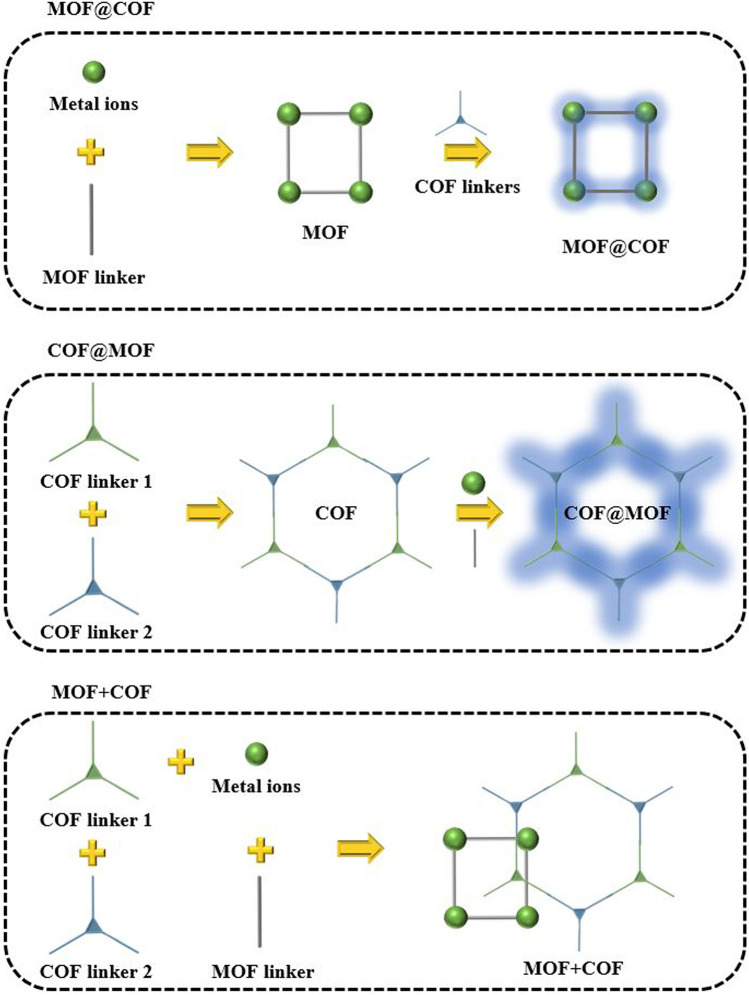
Figure 9.
MOF/COF Hybrid Materials with Multiple Components [203] (CC BY-SA 4.0).
|

|
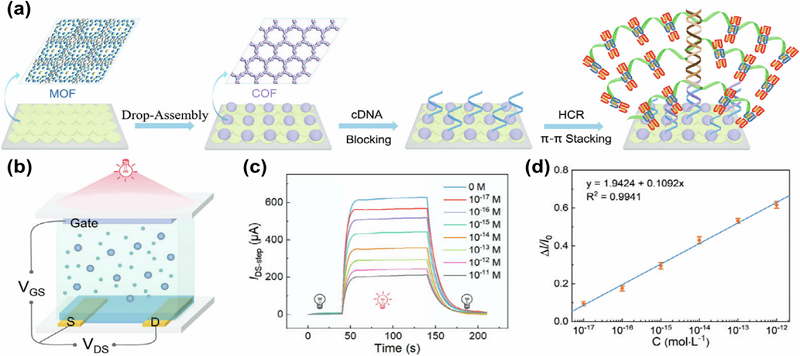
Figure 10.
COF-on-MOF OPECT-based DNA sensors.
a) Gate functionalization process where COF-on-MOF and target DNA trigger GWS growth. b) Schematic of the COF-on-MOF OPECT. c) IDS-step responses of the system to various concentrations of HTLV-II DNA, representing the disparity in values between the currents before and after illumination, and d) the corresponding calibration curve [212] (CC BY-SA 4.0).
|

|
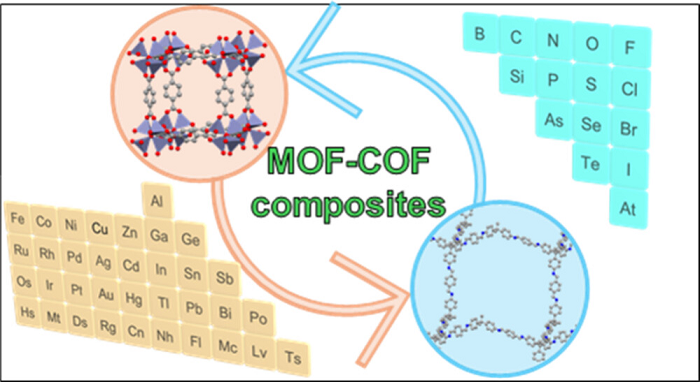
Figure 11.
Formation of MOF-COF and COF–MOF core-shell composites [220] (CC BY-SA 4.0).
|

|
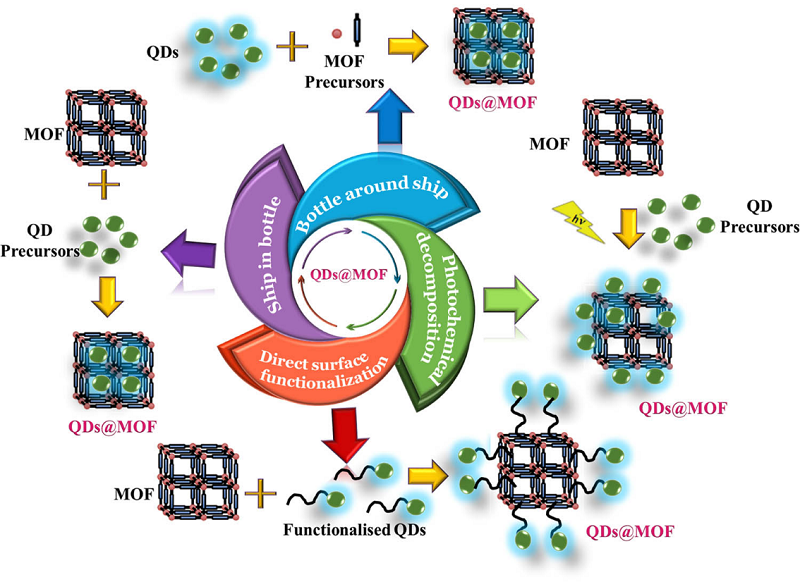
Figure 12.
Schematic representation of various approaches deployed for the preparation of QDs@MOF [229] (CC BY-SA 4.0).
|
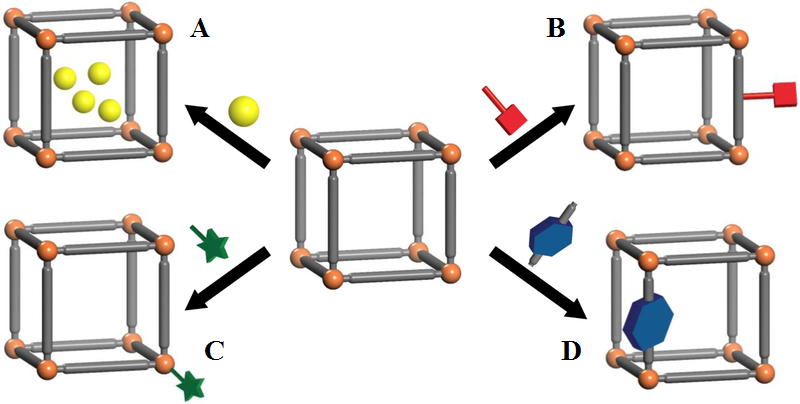

|
Figure 13.
Four common strategies for loading drugs into nMOFs. A - noncovalent encapsulation, B - conjugation to the linkers, C - use of therapeutics as linkers, D - attachment to the SBUs (adapted from [288]).
|
Table 1. Transistors for DNA/RNA sensing [137] (CC BY-SA 4.0).
|
Analyte |
Gate |
Channel material |
Electrolyte |
Detection limit |
Detection range |
Ref. |
|
DNA |
AuNPs/CdS QDs |
PEDOT:PSS |
PBS |
1 fM |
1 fM–1 pM |
|
|
DNA |
AAO-Au |
PEDOT:PBS |
PBS |
0.1 nM |
0.5 nM–12.5 nM |
|
|
DNA |
Au |
PEDOT:PSS |
PBS |
10 pM |
- |
|
|
DNA |
Au/AuNPs |
PEDOT:PSS |
PBS |
0.575 fM |
0.1 pM–1 nM |
|
|
RNA |
6-mercapto-1-hexanol (MCH)/probe/AuNPs/Au |
PEDOT:PSS |
PBS |
2 pM |
5 pM–20 nM |
|
|
DNA |
Carbon/PDA/Probe |
PEDOT:PSS |
PBS |
0.1 pM |
1 pM–10 nM |
|
|
DNA |
Au/probe |
Graphene |
PBS |
1 fM |
1 fM–5 μM |
|
|
RNA |
CdS QDs/TiO2 nanotubes |
PEDOT:PSS |
PBS |
1 pM |
1 pM–10 μM |
|
|
DNA |
ssDNA/CQDs/Au |
Polymethyl methacrylate (PMMA)/graphene |
PBS |
1 aM |
1 aM–0.1 nM |
|
|
RNA |
Au/DNA/miRNA |
PEDOT:PSS |
PBS |
10 pM |
10 pM–1 μM |
|
|
RNA |
Au/ssDNA |
Graphene |
PBS |
10−20 M |
10−20 M–10−12 M |
|
|
RNA |
AuNPs/fluorine-doped tin oxide (FTO)/MCH |
PEDOT:PSS |
PBS |
5.5 fM |
- |
|
|
DNA |
COF-on-MOF |
PEDOT:PSS |
PBS |
0.003 fM |
0.01 fM–1 pM |
|
|
Electrochemical sensors used to detect disease biomarkers |
||||||
|
HIV-1 DNA |
Cu−MOF@CuPc−TA−COF |
DNA hybridization |
Electrochemical signal |
0,18 fM |
1 fM–1 nM |
[156] |
|
Acute myocarditis |
miR-721 |
MOF@Au@G-triplex/hemin nanozyme |
CA |
0.25 fM |
0.5 фM–1 нM |
[157] |
|
Hepatitis C |
HCV gene |
S-BN QDs |
ECL |
0.17 pМ/L |
5 pМ/L - 1 nМ/L |
[158] |
|
Rheumatoid arthritis |
IL-6 |
AuNPs/graphene |
CA |
0.42 pg/ml |
0.97–250 pg/ml |
[159] |
|
Hepatitis B |
HBV-DNA |
BN-CDs |
ECL |
18.08 aM |
100 aM–1 pM |
[160] |
|
HIV |
DNA |
Ni-MOF/AuNPs/CNTs |
DPV |
0.13 nM |
10 nM–1 μM |
[161] |
|
Sepsis |
C-reactive protein (CRP) |
AuNPs@C-ZIF67 |
DPV |
0.44 pg/ml |
10 pg/ml–10 µg/ml |
[162] |
|
Neurodegenerative diseases |
Dopamine |
Ti3C2TxMXene |
ECL |
100 nM |
100 nM ~ 50 мM |
[163] |
|
Covid-2 |
SARS-CoV-2 antigen |
MOF nanohybrids < 5 min |
Electrochemi cal biosensor chip |
6.68 fg/ml (in buffer), 6.20 fg/ml (in serum) |
10–107 fg/ml |
[164] |
|
Breast cancer |
CA15-3 |
GCE/MIL-156 MOF@COF@Au |
DPV |
2.6 nU/мл |
30–100 nU/ml |
[165] |
|
Omicron |
crRNA |
AuE-MXene-AuNPs |
SWV |
1 fM |
1 nM–10 fM |
[166] |
|
AIDS |
HIV DNA |
3D CdSe QDs-DNA/SnO2 nanolowers |
ECL/PEC |
1.38 fM |
0.5 μM–5 fM |
[167] |
|
Ovarian cancer |
CA 125 |
MOF-808/CNTs |
ЕС |
0.5 pg/ml |
0.001~0.1 and 0.1~30 ng/ml |
[168] |
|
Hepatitis B |
HBV DNA |
Al-MOF |
– |
62.1 aM |
100 aM-10 pM |
[169] |
|
Ovarian cancer |
CA125 |
Ce-MOF/TPN-COF/CNT, UiO66@MB |
ЕС |
0, 088 ng/ml |
0,0001-100 U/ml−1 |
[170] |
|
Ovarian cancer |
CA125 |
MXene/MIL-101(Fe)-NH2 |
ЕС |
6,0 ng/ml |
0,2−1000 IU/ml−1 |
[113] |
Table 2. Electrocatalytic performance of various functional molecules based electrochemical biosensors. Adapted from [15].
|
Electrode |
Analyte |
Technique |
Linear Range |
LOD |
Sensitivity |
Ref. |
|
Mg-MOF/GNPs/Mb |
TCA |
CV |
1–200 mM |
0.33 mM |
- |
[123] |
|
Nitrite |
CV |
0.8–18 mM |
0.26 mM |
|||
|
SPE/MoS2-Cu-MOF/GNPs/Ab |
CA125 |
DPV |
0.5 mU/mL –500 U/mL |
0.5 mU |
- |
[124] |
|
Cu-MOF/GNPs/CHA-HCR |
MiR-21 |
DPV |
0.1 fM–100 pM |
0.02 fM |
- |
[125] |
|
GCE/MMOF/GNFs |
H2O2 |
CA |
5 µM–15 mM; |
0.9 µM |
- |
[126] |
|
GCE/FA-Zr-MOF |
HeLa cells |
EIS |
1 × 102–1 ×106 cells/mL |
90 cells/mL |
- |
[127] |
|
GCE/NA-Zr-MOF/dsDNA |
MiR-21 |
DPV |
0.02–10 pM |
8.2 fM |
- |
[128] |
|
Let-7a |
0.01–10 pM |
3.6 fM |
||||
|
Cr-MOF/PdNPs/c-DNA |
HeLa cells |
DPV |
5 × 102–1.62 ×107 cells/mL |
11.25 cells/mL |
- |
[129] |
|
Co-MOF/GNPs/MWCNT/Cyt-c |
nitrite |
DPV |
5 nM–1 mM |
4.4 nM |
- |
[131] |
|
ITO/PANI/Cu-MOF/Ab |
E. coli |
EIS |
2–2 × 108 cfu/mL |
2 cfu/mL |
- |
[132] |
|
SPE/TCOF/SOD |
Superoxide |
CA |
10 nM–100 µM |
0.5 nM |
- |
[138] |
|
GCE/COF/ACLE |
Carbaryl |
EIS |
0.48–35 µM |
0.16 µM |
- |
[139] |
|
GCE/Fe3O4COF/HRP |
HQ |
DPV |
0.5–300 µM |
120 nM |
- |
[140] |
|
GCE/Fe3O4COF/GNPs/DNA |
ATP |
CV |
5 pM–50 µM |
1.6 pM |
- |
[141] |
|
COF/DNA/HRP |
exosomes |
CA |
104– 107 particles/µL |
7668 particles/µL |
- |
[142] |
Table 3. The detection performances of carbon nanomaterials-decorated MOF−based electrochemical sensors [251] (CC BY-SA 4.0).
|
Electrode Material |
Analyte |
Linear Range |
LOD |
|
MIL-101(Cr)@rGO |
4-nonylphenol |
100 nM~12.5 μM |
33 nM |
|
Cu−hemin MOF/CS−rGO |
H2O2 |
65 nM~0.41 mM |
19 nM |
|
Cu(tpa)−EGR |
ACOP and DA |
1.0 μM~100 μM and 1.0 μM~50 μM |
0.36 and 0.21 μM |
|
Co−MOF/BP−RGO |
chlorogenic acid |
1.0 nM~391 μM |
14 nM |
|
PPy@ZIF-8/GAs |
dichlorophenol |
10 nM~10 μM |
0.1 nM |
|
GA-UiO-66-NH2 |
Cd2+, Pb2+, Cu2+, and Hg2+ |
10 nM~1.5 μM, 1.0 nM~2.0 μM, |
9.0, 1.0, 8.0 and |
|
Ni−MOFs/CNTs |
H2O2 |
10 μM~51.6 mM |
2.1 μM |
|
UiO-66-NH2/CNTs |
DA and AC |
30 nM~2.0 μM |
15 and 9.0 nM |
|
Mn−BDC@MWCNT |
AA, DA and UA |
0.1 μM~1.15 mM, 10 nM~0.5 mM, and 20 nM~1.1 mM |
10, 2.0 and 5.0 nM |
|
3D Ni−MOF@CNTs |
BPA |
1.0 nM~1.0 μM |
0.35 nM |
|
MB@MWCNTs/UiO-66-NH2 |
doxorubicin |
0.1 μM~75 μM |
51 nM |
|
Co−MOF@MPC |
hydrazine and |
5.0 μM~0.63 mM and 0.5 μM~15 μM |
1.75 and 0.21 μM |
|
ZIF67−OMC |
HQ and catechol |
0.1 μM~100 μM |
52 and 36 nM |
|
DUT-9/MC |
Baicalein |
50 nM~20 μM |
15 nM |
|
pFeMOF/OMC |
H2O2 |
0.5~70.5 μM |
0.45 μM |
|
Cu−MOFs/OMC |
hydrazine |
0.5 μM~0.711 mM |
0.35 μM |
|
Ag−ZIF-8/OMC |
xanthine |
1.0 μM~0.28 mM |
0.167 μM |
|
MOF-808/CNTs |
CA 125 |
0.001~0.1 and 0.1~30 ng/mL |
0.5 pg/mL |
|
PDA/ZIF-8@rGO |
Glucose |
1.2 μM~1.2 mM |
0.333 μM |
|
GDH/MG−Tb@MOF−CNTs |
Glucose |
25 μM~17 mM |
8 μM |
|
HRP/ZIF-67(Co)/MWCNT |
H2O2 |
1.86~1050 μM |
0.11 μM |
Abbreviation: rGO, reduced graphene oxide; MOFs, metal–organic framework; CS, chitosan; tpa, terephthalate; EGR, electrochemically reduced graphene; ACOP, acetaminophen; PPy, polypyrrole; GA, graphene aerogel; CNTs, carbon nanotubes; MWCNT, multiwall carbon nanotubes; MB, methylene blue; MPC, mesoporous carbon; OMC, ordered mesoporous carbon; HQ, hydroquinone.
Table 4. COF–MOF hybrids for extraction and determination of water contaminants (adapted from [335]).
|
Hybrid materials |
Contaminants |
Determination methods |
Detection limits |
Linear ranges |
|
NH2-MIL-68(In)@COF |
SAs |
HPLC-VWD |
1 ng mL−1 |
10–2000 ng mL−1 |
|
m-NH2-MIL-68(In)@COF |
SAs |
MSPE-HPLC |
0.1–0.5 ng mL−1 |
10–2000 ng mL−1 |
|
Fe3O4@TbBD@ZIF-8 |
Sedatives |
MSPE-HPLC/MS/MS |
0.04-0.2 µg k−1 g |
0.03-70 µg k−1 g |
|
MOFL-TpBD |
TNP |
Fluorescence |
3.52 × 10−6 m |
0.01–1 × 10−3 m |
|
MOFL-TpBD |
Pb2+ |
SPE |
0.32 µg L−1 |
0.7–12 µg L−1 |
|
UiO-66@COF-V |
PCAs |
d-SPE-LC-MS/MS |
0.69-1.79 ng L−1 |
10–1000 ng L−1 |
|
CCF@NH2-UiO-66@TpBD |
BPs |
PT-SPE-UPLC |
0.16-0.75 ng mL−1 |
– |
|
Fe3O4@NH2-UiO-66@TbBD-COF |
Cu2+ |
MSPE-UV–vis |
0.0376 × 10−6 m |
0.05–1.2 × 10−6 m |
|
UiO@TapbTp |
Aspirin |
MALDI-TOF MS |
0.001 mg L−1 |
0.05–5 mg L−1 |
|
UiO@TapbTp |
Ketoprofen |
MALDI-TOF MS |
0.001 mg L−1 |
0.01–5 mg L−1 |
|
UiO@TapbTp |
Naproxen |
MALDI-TOF MS |
0.010 mg L−1 |
0.10–5 mg −1L |
|
Ce-MOF@COF |
OTC |
Electrochemical sensing |
17.4 fg mL−1 |
0.1–0.5 ng mL−1 |
|
Co-MOF@TPN-COF |
AMP |
Electrochemical sensing |
0.217 fg mL−1 |
1.0 fg mL−1–2.0 ng mL−1 |
|
UC-1 |
F− |
Fluorescence sensing |
– |
0-500 × 10−6 m |
FUNDING
The work was carried out within the framework of the state assignment of the Ministry of Science and Higher Education of the Russian Federation “Medical and biological study of boron-containing nanoparticles and bionanoconstructions for diagnostics and boron-neutron capture therapy of superficial malignant tumors” project (No, 1024011000011-7-1.4.2;3.5.2.)
REFERENCES
- Mohammad, R. S., Navid, R., Masoud, M., Francis, V., Leonid, G. V., Rafael,L. (2021) Metal-organic frameworks (MOFs) for cancer therapy. Materials,14(23), 7277. DOI
- Kargozar, S., Hoseini, S. J., Milan, P. B., Hooshmand, S., Kim, H.-W.,Mozafari, M. (2020) Quantum Dots: A Review from Concept to Clinic. SpecialIssue: AFOB XV – Nanomaterials for Biomedical Applications, 15(12),2000117. DOI
- Liu, S.-L., Wang, Z.-G., Xie, H.-Y., Liu, An-An, Lamb, D. C., Pang,D.-W. (2020) Single-Virus Tracking: From Imaging Methodologies toVirological Applications. Chem. Rev., 120, 1936−1979. DOI
- Rehan, F., Zhang, M., Fang, J., Greish, K. (2024) Therapeutic Applicationsof Nanomedicine: Recent Developments and Future Perspectives. Molecules,29(9), 2073. DOI
- Cote, A. P., Benin, A. I., Ockwig, N. W., O’Keeffe, M., Matzger, A. J., Yaghi,O. M. (2005) Porous, crystalline, covalent organic frameworks. Science, 310,1166–1170. DOI
- Wu, J., Liu, H., Chen, W., Ma, B., Ju, H. (2023) Device integration ofelectrochemical biosensors. Nat. Rev. Bioeng, 1(5), 346-360. DOI
- Li, X., Zheng, X., Yuan, Y., Deng, J., Su, L., Xu, K. (2025) A review ofresearch progress on COF-based biosensors in pathogen detection. Anal. Chim.Acta, 1342, 343605. DOI
- Afshariazar, F., Morsali, A. (2021) A dual-response regenerable luminescent2D-MOF for nitroaromatic sensing via target-modulation of active interactionsites. J. Mater. Chem. C, 9, 12849–12858. DOI
- Huo, Y. P., Liu, S., Gao, Z. X., Ning, B. A., Wang, Y. (2021) State-of-the-artprogress of switch fluorescence biosensors based on metal-organic frameworksand nucleic acids. Mikrochim Acta, 188(5), 168. DOI
- Wang, X., Ye, N. (2017) Recent advances in metal-organic frameworksand covalent organic frameworks for sample preparation and chromatographicanalysis. Electrophoresis, 38(24), 3059-3078. DOI
- Zuliani, A., Khiar, N., Carrillo-Carrión, C. (2023) Recent progress ofmetal–organic frameworks as sensors in (bio)analytical fields: towards realworldapplications. Anal. Bioanal. Chem., 415, 2005–2023. DOI
- Liang, H., Wang, L., Yang, Y., Song, Y., Wang, L. (2021) A novelbiosensor based on multienzyme microcapsules constructed from covalentorganicframework. Biosens. Bioelectron., 193, 113553. DOI
- Yue, Y., Ji, D., Liu, Y., Wei, D. (2024) Chemical Sensors Based onCovalent Organic Frameworks. Chemistry, 30(3), e202302474. DOI
- Păun, C., Motelică, L., Ficai, D., Ficai, A., Andronescu, E. (2023) Metal-Organic Frameworks: Versatile Platforms for Biomedical Innovations. Materials(Basel), 16(18), 6143. DOI
- Theyagarajan, K., Kim, Y. J. (2023) Recent Developments in the Designand Fabrication of Electrochemical Biosensors Using Functional Materials andMolecules. Biosensors (Basel), 13(4), 424. DOI
- Deng, Y., Wang, Y., Xiao, X., Saucedo, B. J., Zhu, Z., Xie, M., Xu, X., Yao,K., Zhai, Y., Zhang, Z., Chen, J. (2022) Progress in Hybridization of CovalentOrganic Frameworks and Metal-Organic Frameworks. Small, 18(38), e2202928. DOI
- Saboorizadeh, B., Zare-Dorabei, R., Safavi, M., Safarifard, V. (2024)Applications of Metal-Organic Frameworks (MOFs) in Drug Delivery,Biosensing, and Therapy: A Comprehensive Review. Langmuir., 40(43), 22477-22503. DOI
- Moghadam, P. Z. Li, A. Wiggin, S. B. Tao, A. Maloney, A. G. P. Wood,P. A. Ward, S. C. Fairen-Jimenez, D. (2017) Development of a CambridgeStructural Database Subset: A Collection of Metal–Organic Frameworks forPast, Present, and Future. Chem. Mater, 29, 2618–2625. DOI
- Majumdar, S., Moosavi, S. M., Jablonka, K. M., Ongari, D., Smit, B. (2021)Diversifying Databases of Metal Organic Frameworks for High-ThroughputComputational Screening. ACS Appl. Mater. Interfaces, 13, 61004–61014. DOI
- Wang, Q., Sun, Y., Li, S., Zhang, P., Yao, Q. (2020) Synthesis andmodification of ZIF-8 and its application in drug delivery and tumor therapy.RSC Adv., 10, 37600-37620. DOI
- Dutta, A., Pan, Y., Liu, J.Q., Kumar, A. (2021) Multicomponent isoreticularmetal-organic frameworks: Principles, current status and challenges. Coord.Chem. Rev., 445, 214074. DOI
- Wang, B., Cote, A. P., Furukawa, H., O’Keeffe, M., Yaghi, O. M. (2002)Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxidereservoirs. Nature, 453(7192), 207–211. DOI
- Latroche, M., Surble, S., Serre, C., Mellot-Draznieks, C., Llewellyn, P. L.,Lee, J. H., Chang, J. S., Jhung, S. H., Ferey, G. (2006) Hydrogen storage in thegiant-pore metal-organic frameworks MIL-100 and MIL-101. Angew. Chem.Int. Ed. Engl, 45(48), 8227–8231. DOI
- Ma, S., Sun, D., Simmons, J. M., Collier, C. D., Yuan, D. Q., Zhou, H. C.(2008) Metal-organic framework from an anthracene derivative containingnanoscopic cages exhibiting high methane uptake. J. Am. Chem. Soc, 130(3),1012–1016. DOI
- Cavka, J. H., Jakobsen, S., Olsbye, U., Guillou, N., Lamberti, C., Bordiga,S., Lillerud, K. P. (2008) A new zirconium inorganic building brick formingmetal organic frameworks with exceptional stability. J. Am. Chem. Soc,130(42), 13850–13851. DOI
- Jiao, L., Seow, J. Y. R., Skinner, W. S., Wang, Z. U., Jiang, H. L. (2019)Metal-organic frameworks: structures and functional applications. Mater. Today,27, 43–68. DOI
- Pashazadeh-Panahi, P., Belali, S., Sohrabi, H., Oroojalian, F., Hashemzaei,M., Mokhtarzadeh, A., de la Guardia, M. (2021) Metal-organic frameworksconjugated with biomolecules as efficient platforms for development ofbiosensors. TrAC Trends Anal. Chem., 141, 116285. DOI
- Jassal, A. K., Kajal, P. (2024). Quantum Dots@Metal–Organic FrameworksComposites. In: Thomas, S., Das, P., Ganguly, S. (eds) Quantum Dots BasedNanocomposites. Engineering Materials. Springer, Cham. DOI
- Jia, J., Zhang, S., Wen, K., Li, Q. (2019) Nano-scaled zeolitic imidazoleframework-8 as an efficient carrier for the intracellular delivery of RNase Ain cancer treatment. Int. J. Nanomedicine, 14, 9971–9981. DOI
- Teplensky, M. H., Fantham, M., Poudel, C., Hockings, C., Lu, M., Guna,A., Aragones-Anglada, M., Moghadam, P. Z., Li, P., Farha, O. K., Bernaldo deQuirós, F. S., Richards, F. M., Jodrell, D. I., Kaminski, S. G., Kaminski, C. F.,Fairen-Jimenez, D. (2019) A highly porous metal-organic framework systemto deliver payloads for gene knockdown. Chem., 5(11), 2926–2941. DOI
- Shi, L., Wu, J., Qiao, X., Ha, Y., Li, Y., Peng, C., Wu, R. (2020) In situbiomimetic mineralization on ZIF-8 for smart drug delivery. ACS Biomater. Sci.Eng, 6(8), 4595–4603. DOI
- Zhang, Y., Lai, L., Liu, Y., Chen, B., Yao, J., Zheng, P., Pan, Q., Zhu, W.(2022) Biomineralized cascade enzyme-encapsulated ZIF-8 nanoparticlescombined with antisense oligonucleotides for drug-resistant bacteria treatment.ACS Appl. Mater. Interfaces, 14(5), 6453–6464. DOI
- Abdelhamid, H. N., Dowaidar, M., Langel, Ü. (2020) Carbonized chitosanencapsulated hierarchical porous zeolitic imidazolate frameworks nanoparticlesfor gene delivery. Microporous Mesoporous Mater, 302, 110200. DOI
- Khalilian, S.F., Tohidi, M., Rastegari, B. (2020) Synthesis of abiocompatible nanoporous zeolitic imidazolate framework-8 in the presence ofGum Arabic inspired by the biomineralization process. CrystEngComm, 22(10),1875–1884. DOI
- Ren, L., Xiao, X., Chen, Y., Yu, Y., Zhang, Q., Liu, R., Xu, W. (2019)Preparation of ZIF-8/natural plant fiber composites via biomimeticmineralization for highly efficient removal of formaldehyde. ChemistrySelect,4(42), 12294–12303. DOI
- Velásquez-Hernández, M. J., Astria, E., Winkler, S., Liang, W., Wiltsche, H.,Poddar A., Shukla R., Prestwich G., Paderi J., Salcedo-Abraira P., AmenitschH., Horcajada P., Doonan, C. J., Falcaro, P. (2020) Modulation of metalazolateframeworks for the tunable release of encapsulated glycosaminoglycans.Chem Sci., 11(39), 10835–10843. DOI
- Li, S., Dharmarwardana, M., Welch, R. P., Ren, Y., Thompson, C. M.,Smaldone R. A., Gassensmith, J. J. (2016) Template-directed synthesis ofporous and protective core-shell bionanoparticles. Angew. Chem. Int. Ed. Engl,55(36), 10691–10696. DOI
- Liang, K., Richardson, J. J., Cui, J., Caruso, F., Doonan, C. J., Falcaro, P.(2016) Metal–organic framework coatings as cytoprotective exoskeletons forliving cells. Adv. Mater, 28(36), 7910–7914. DOI
- Liang, K., Richardson, J. J., Doonan, C. J., Mulet, X., Ju, Y., Cui, J.,Caruso, F., Falcaro, P. (2017) An enzyme-coated metal–organic frameworkshell for synthetically adaptive cell survival. angewandte chemie internationaledition. Angew. Chem. Int. Ed. Engl, 56(29), 8510–8515. DOI
- Li, Y., Zhang, K., Liu, P., Chen, M., Zhong, Y., Ye, Q., Wei, M. Q., Zhao, H.,Tang, Z. (2019) Encapsulation of plasmid DNA by nanoscale metal–organicframeworks for efficient gene transportation and expression. Adv. Mater,31(29), e1901570. DOI
- Polash, S. A., Garlick-Trease, K., Pyreddy, S., Periasamy, S., Bryant,G., Shukla, R. (2023) Amino acid-coated zeolitic imidazolate framework fordelivery of genetic material in prostate cancer cell. Molecules, 28(12), 4875. DOI
- Alyami, M. Z., Alsaiari, S. K., Li, Y., Qutub, S. S., Aleisa, F.A., Sougrat,R., Merzaban, J. S., Khashab, N. M. (2020) Cell-type-specific CRISPR/Cas9delivery by biomimetic metal organic frameworks. J. Am. Chem. Soc, 142(4),1715–1720. DOI
- Alsaiari, S. K., Patil, S., Alyami, M., Alamoudi, K. O., Aleisa, F. A.,Merzaban, J. S., Li, M., Khashab, N. M. (2018) Endosomal escape and deliveryof CRISPR/Cas9 genome editing machinery enabled by nanoscale zeoliticimidazolate framework. J. Am. Chem. Soc, 140(1), 143–146. DOI
- Liu, C., Xu, X., Koivisto, O., Zhou, W., Jacquemet, G., Rosenholm, J. M.,Zhang, H. (2021) Improving the knock-in efficiency of the MOF-encapsulatedCRISPR/Cas9 system through controllable embedding structures. Nanoscale,13(39), 16525–16532. DOI
- Poddar, A., Pyreddy, S., Carraro, F., Dhakal, S., Rassell, A., Field, M. R.,Reddy, T. S., Falcaro, P., Doherty, C. M., Shukla, R. (2020) ZIF-C for targetedRNA interference and CRISPR/Cas9 based gene editing in prostate cancer.Chem. Commun. (Camb), 56(98), 15406–15409. DOI
- Lee, H. J., Wark, A. W., Corn, R. M. (2008) Microarray methods for proteinbiomarker detection. Analyst, 133, 975. DOI
- Tran, V. A., Le, V. T., Doan, V. D., Giang, N. L. Vo. (2023) Utilization ofFunctionalized Metal-Organic Framework Nanoparticle as Targeted DrugDelivery System for Cancer Therapy. Pharmaceutics, 15(3), 931. DOI
- Smith, G. P. (1985) Filamentous fusion phage: novel expression vectors thatdisplay cloned antigens on the virion surface. Science, 228(4705), 1315-1317. DOI
- Petrenko, V. A., Smith, G. P. (2000) Phages from landscape libraries assubstitute antibodies. Protein Eng, 13, 589–592. DOI
- Zhang, W., Arramel, A., Wong, P. K. J., Zhang, L., Zheng, J., Zhang, W.,Zhang, H., Yan, X., Qi, J., Li, J. (2020) Core–shell hybrid zeolitic imidazolateframework-derived hierarchical carbon for capacitive deionization. J. Mater.Chem. A, 8, 14653–14660. DOI
- Biswal, B. P., Shinde, D. B., Pillai, V. K., Banerjee, R. (2013) Stabilizationof graphene quantum dots (GQDs) by encapsulation inside zeolitic imidazolateframework nanocrystals for photoluminescence tuning. Nanoscale, 5, 10556–10561. DOI
- Reali, S., Najib, E. Y., Treuerné Balázs, K. E., Tan, A. C. H., Váradi, L.,Hibbs, D. E., Groundwater, P. W. (2019) Novel diagnostics for point-of-carebacterial detection and identification. RSC Adv, 9, 21486-21497. DOI
- Davydova, A., Vorobjeva, M., Pyshnyi, D., Altman, S., Vlassov, V.,Venyaminova, A. (2016) Aptamers against pathogenic microorganisms. Crit.Rev. Microbiol, 42(6), 847–865. DOI
- Anderson, G. P., Glaven, R. H., Algar, W. R., Susumu, K., Stewart, M. H.,Medintz, I. L., Goldman, E. R. (2013) Single domain antibody–quantum dotconjugates for ricin detection by both fluoroimmunoassay and surface plasmonresonance. Anal. Chim. Acta, 786, 132–138. DOI
- Fetter, L., Richards, J., Daniel, J., Roon, L., Rowland, T. J., Bonham, A. J.(2015) Electrochemical aptamer scaffold biosensors for detection of botulismand ricin toxins. Chem. Commun., 51, 15137–15140. DOI
- Lamont, E. A., He, L. L., Warriner K., Labuza, T. P., Sreevatsan, S. (2011) Asingle DNA aptamer functions as a biosensor for ricin. Analyst, 136, 3884–3895. DOI
- Guryev, E.L., Shanwar, S., Zvyagin, A.V., Deyev, S.M., Balalaeva, I.V.(2021) Photoluminescent Nanomaterials for Medical Biotechnology. ActaNaturae, 13(2), 16-31. DOI
- Park, J. W., Lee, S. J., Choi, E. J., Kim, J., Song, J. Y., Gu, M. B. (2014)An ultra-sensitive detection of a whole virus using dual aptamers developedby immobilization-free screening. Biosens. Bioelectron., 51, 324-329. DOI
- Tumanov, Yu.V., Boldyrev, A.N., Autenshlyus, A.I. Medical biotechnology:diagnostics of diseases and development of drugs, NGTU, Novosibirsk, 2016,214 pp.
- Bi, S., Yue, S., Zhang, S. (2017) Hybridization chain reaction: a versatilemolecular tool for biosensing, bioimaging, and biomedicine. Chem. Soc. Rev.,46(14), 4281-4298. DOI
- Bardajee, G. R., Zamani, M., Mahmoodian, H., Elmizadeh, H., Yari,H., Jouyandeh, L., Shirkavand, R., Sharifi, M. (2022) Capability of novelfluorescence DNA-conjugated CdTe/ZnS quantum dots nanoprobe forCOVID-19 sensing. Spectrochim. Acta A. Mol. Biomol. Spectrosc, 269,120702. DOI
- Hötzer, B., Medintz, I. L., Hildebrandt, N. (2012) Fluorescence inNanobiotechnology: Sophisticated Fluorophores for Novel Applications. Small,8, 2297. DOI
- Dasilva, N., Díez, P., Matarraz, S., González-González, M., Paradinas, S.,Orfao, A., Fuentes, M. (2012) Biomarker Discovery by Novel Sensors Based onNanoproteomics Approaches. Sensors, 12, 2284. DOI
- Sandana Mala, J. G., Rose, C. (2014) Facile production of ZnS quantumdot nanoparticles by Saccharomyces cerevisiae MTCC 2918. J. Biotechnol, 170,73–78. DOI
- Zorab, M. M., Mohammadjani, N., Ashengroph, M., Alavi, M. (2023)Biosynthesis of Quantum Dots and Their Therapeutic Applications in theDiagnosis and Treatment of Cancer and SARS-CoV-2. Adv. Pharm. Bull, 13(3),411–422. DOI
- Dabbousi, B. O., Rodriguez-Viejo, J., Mikulec, F. V., Heine, J. R.,Mattoussi, H., Ober, R., Jensen, K. F., Bawendi, M. G. (1997) (CdSe) ZnScore−shell quantum dots: synthesis and characterization of a size series ofhighly luminescent nanocrystallites. J. Phys. Chem., B, 101, 9463–9475. DOI
- Wang, J., Mora-Seró, I., Pan, Z., Zhao, K., Zhang, H., Feng, Y., Yang, G.,Zhong, X., Bisquert, J. (2013) Core/shell colloidal quantum dot exciplex statesfor the development of highly efficient quantum-dot-sensitized solar cells. J.Am. Chem. Soc, 135, 15913. DOI
- Kaur, A., Dhakal, S. (2020) Recent applications of FRET-based multiplexedtechniques. Trac-Trends Anal. Chem., 123, 115777. DOI
- Racca, L., Cauda, V. (2021) Remotely Activated Nanoparticles forAnticancer Therapy. Nano-Micro Lett, 13, 11. DOI
- Lidke, D. S., Nagy, P., Heintzmann, R., Arndt-Jovin, D. J., Post, J. N.,Grecco, H. E., Jares-Erijman, E. A., Jovin, T. M. (2004) Quantum Dot LigandsProvide New Insights into erbB/HER Receptor−Mediated Signal Transduction.Nat. Biotechnol, 22, 198−203. DOI
- Srinivasan, C., Lee, J., Papadimitrakopoulos, F., Silbart, L. K., Zhao, M.,Burgess, D. J. (2006) Labeling and Intracellular Tracking of Functionally ActivePlasmid DNA with Semiconductor Quantum Dots. Mol. Ther, 14, 192−201. DOI
- Wu, X., Liu, H., Liu, J., Haley, K. N., Treadway, J. A., Larson, J. P., Ge, N.,Peale, F., Bruchez, M. P. (2003) Immunofluorescent Labeling of Cancer MarkerHer2 and Other Cellular Targets with Semiconductor Quantum Dots. Nat.Biotechnol, 21, 41−46. DOI
- Chen, C., Peng, J., Xia, H., Wu, Q., Zeng, L., Xu, H., Tang, H., Zhang,Z., Zhu, X., Pang, D., et al. (2010) Quantum-Dot-Based ImmunofluorescentImaging of HER2 and ER Provides New Insights into Breast CancerHeterogeneity. Nanotechnology, 21, 095101. DOI
- Chen, C., Xia, H. S., Gong, Y. P., Peng, J., Peng, C. W., Hu, M. B., Zhu, X.B., Pang, D. W., Sun, S. R., Li, Y. (2010) The Quantitative Detection of TotalHER2 Load by Quantum Dots and the Identification of a New Subtype ofBreast Cancer with Different 5-Year Prognosis. Biomaterials, 31, 8818−8825. DOI
- Chen, C., Liu, S. L., Cui, R., Huang, B. H., Tian, Z. Q., Jiang, P., Pang, D.W., Zhang, Z. L. (2008) Diffusion Behaviors of Water-Soluble CdSe/ZnS Core/Shell Quantum Dots Investigated by Single-Particle Tracking. J. Phys. Chem. C,112(48), 18904−18910. DOI. org/10.1021/jp807074t
- Gao, X., Wang, T., Wu, B., Chen, J., Chen, J., Yue, Y., Dai, N., Chen, H.,Jiang, X. (2008) Quantum Dots for Tracking Cellular Transport of Lectin-Functionalized Nanoparticles. Biochem. Biophys. Res. Commun., 377, 35−40. DOI
- Liu, S.-L., Wang, Z.-G., Xie, H.-Y., Liu, A.-A., Lamb, D. C., Pang,D.-W. (2020) Single-Virus Tracking: From Imaging Methodologies toVirological Applications. Chem. Rev., 120, 1936−1979. DOI
- Kargozar, S., Hoseini, S. J., Milan, P. B., Hooshmand, S., Kim, H.-W.,Mozafari, M. (2020) Quantum Dots: A Review from Concept to Clinic.Biotechnol. J., 15(12), e2000117. DOI
- Lim, J., Bae, W. K., Kwak J., Lee, S., Lee, C., Char, K. (2012) Towardszero-threshold optical gain using charged semiconductor quantum dots. Optical.Mater. Express, 2, 594-698. DOI
- Vasil’ev, R. B., Dirin, D. N. Kvantovye tochki: sintez, svojstva, primenenie,Metodicheskie materialy. MGU im. M.V. Lomonosova: Moskva, 2007. 34 s.
- Poddar, A., Conesa, J. J., Liang, K., Dhakal, S., Reineck, P., Bryant, G.,Pereiro, E., Ricco, R., Amenitsch, H., Doonan, C., Mulet, X., Doherty, C. M.,Falcaro, P., Shukla, R. (2019) Encapsulation, visualization and expression ofgenes with biomimetically mineralized zeolitic imidazolate framework-8 (ZIF-8). Small, 15(36), e1902268. DOI
- Maysinger, D., Ji, J., Hutter, E., Cooper, E. (2015) Nanoparticle-Based andBioengineered Probes and Sensors to Detect Physiological and PathologicalBiomarkers in Neural Cells. Front. Neurosci, 9, 480. DOI
- Liu, T., Xing, R., Zhou, Y.-F., Zhang, J., Su, Y.-Y., Zhang, K.-Q., He, Y.,Sima, Y.-H., Xu, S.-Q. (2014) Hematopoiesis toxicity induced by CdTe quantumdots determined in an invertebrate model organism. Biomaterials, 35, 2942. DOI
- Xu, G., Zeng, S., Zhang, B., Swihart, M. T., Yong, K.-T., Prasad, P. N.(2016) New Generation Cadmium-Free Quantum Dots for Biophotonics andNanomedicine. Chem. Rev., 116, 12234. DOI
- Khan, Z. U., Khan, L. U., Brito, H. F., Gidlund, M., Malta, O. L., DiMascio, P. (2023) Colloidal Quantum Dots as an Emerging Vast Platform andVersatile Sensitizer for Singlet Molecular Oxygen Generation. ACS Omega,8(38), 34328-34353. DOI
- Liu, S.-L., Wang, Z.-G., Xie, H.-Y., Liu, A.-A., Lamb, D. C., Pang,D.-W. (2020) Single-Virus Tracking: From Imaging Methodologies toVirological Applications. Chem. Rev., 120(3), 1936–1979. DOI
- Bilan, R., Nabiev, I., Sukhanova, A. (2016) Quantum Dot-Based Nanotoolsfor Bioimaging, Diagnostics, and Drug Delivery. Chembiochem, 17(22), 2103-2114. DOI
- Srinivasan, C., Lee, J., Papadimitrakopoulos, F., Silbart, L. K., Zhao, M.,Burgess, D. J. (2006) Labeling and intracellular tracking of functionally activeplasmid DNA with semiconductor quantum dots. Mol. Ther, 14, 192–201. DOI
- Shirahata, N. Nanoparticle Biomarkers Adapted for Near-InfraredFluorescence Imaging. In: Wakayama, Y., Ariga, K. (eds) System-MaterialsNanoarchitectonics. NIMS Monographs. Springer: Tokyo, 2022. DOI
- Păun, C., Motelică, L., Ficai, D., Ficai, A., Andronescu, E. (2023) Metal-Organic Frameworks: Versatile Platforms for Biomedical Innovations. Materials(Basel), 16(18), 6143. DOI
- Alli, U., Hettiarachchi, S., Kellici, S. (2020) Chemical Functionalisation of2D Materials by Batch and Continuous Hydrothermal Flow Synthesis. Chem.–Eur. J., 26, 6447–6460. DOI
- Abderrahmane, A., Woo, C., Ko, P.-J. (2022) Low Power ConsumptionGate-Tunable WSe2/SnSe2 van der Waals Tunnel Field-Effect Transistor.Electronics, 11(5), 833. DOI
- Abderrahmane, A., Jung, P.-G., Woo, C., Ko, P. J. (2022) Effect of GateDielectric Material on the Electrical Properties of MoSe2-Based Metal–Insulator–Semiconductor Field-Effect Transistor. Crystals, 12(9), 1301. DOI
- Vu, C-A., Chen, W-Y. (2019) Field-effect transistor biosensors forbiomedical applications: recent advances and future prospects. Sensors, 19(19),4214. DOI
- Vu, C. A., Chen, W. Y. (2020) Predicting Future Prospects of Aptamersin Field-Effect Transistor Biosensors. Molecules, 25(3), 680. DOI
- Syedmoradi, L., Ahmadi, A., Norton, M. L., Omidfar, K. (2019) A review onnanomaterial-based field effect transistor technology for biomarker detection.Mikrochim Acta, 186(11), 739. DOI
- Panahi, A., Sadighbayan, D., Forouhi, S., Ghafar-Zadeh, E. (2021) RecentAdvances of Field-Effect Transistor Technology for Infectious Diseases.Biosensors (Basel), 11(4), 103. DOI
- Fazio, E., Spadaro, S., Corsaro, C., Neri, G, Leonardi, S. G., Neri, F.,Lavanya, N., Sekar, C., Donato, N., Neri, G. (2021) Metal-Oxide BasedNanomaterials: Synthesis, Characterization and Their Applications in Electricaland Electrochemical Sensors. Sensors (Basel), 21(7), 2494. DOI
- Bungon, T., Haslam, C., Damiati, S., O’Driscoll, B., Whitley, T., Davey,P., Siligardi, G., Charmet, J., Awan, S. A. (2021) Graphene FET Sensors forAlzheimer’s Disease Protein Biomarker Clusterin Detection. Front. Mol.Biosci., 8, 651232. DOI
- Ivanov, Yu. D., Pleshakova, T. O., Kozlov, A. F., Malsagova, K. A., Krohin,N. V., Shumyantseva, V. V., Shumov, I. D., Popov, V. P., Naumova, O. V., Fomin,B. I., Nasimov, D. A., Aseev, A. L., Archakov, A. I. (2012) SOI nanowire for thehigh-sensitive detection of HBsAg and α-fetoprotein. Lab on a Chip, 12(23),5104-5111. DOI
- Zhang, G.-J., Zhang, L., Huang, M. J., Luo, Z. H. H., Tay, G. K. I., Lim,E.-J. A., Kang, T. G., Chen Y. (2010) Silicon nanowire biosensor for highlysensitive and rapid detection of Dengue virus. Sens. Actuators B, 146, 138–144. DOI
- Su, P.-C., Chen, B.-H., Lee, Y.-C., Yang, Y.-S. (2020) Silicon NanowireField-Effect Transistor as Biosensing Platforms for Post-TranslationalModification. Biosensors, 10, 213. DOI
- Jin, Q., Men, K., Li, G., Ou, T., Lian, Z., Deng, X., Zhao, H., Zhang, Q.,Ming, A., Wei, Q., Wei, F., Tu, H. (2024) Ultrasensitive Graphene Field-EffectBiosensors Based on Ferroelectric Polarization of Lithium Niobate for BreastCancer Marker Detection. ACS Appl. Mater. Interfaces, 16(22), 28896–28904. DOI
- Xu, B. Z., Zhu, M. S., Zhang, W. C., Zhen, X., Pei, Z. X., Xue, Q., Zhi C.Y., Shi, P. (2016) Ultrathin MXene-Micropattern-Based Field-Effect Transistorfor Probing Neural Activity. Adv. Mater, 28, 3333–3339. DOI
- Mostafavi, E., Iravani, S. (2022) MXene-Graphene Composites: APerspective on Biomedical Potentials. Nanomicro Lett, 14(1), 130. DOI
- Li, Y., Peng, Z, Holl, N. J., Hassan, M. R., Pappas, J. M., et al. (2021)MXene-graphene field-effect transistor sensing of influenza virus and SARSCoV-2. ACS Omega, 6(10), 6643–6653. DOI
- Gu, H., Xing, Y., Xiong, P., Tang, H., Li, C., et al. (2019) Threedimensionalporous Ti3C2Tx MXene-graphene hybrid films for glucosebiosensing. ACS Appl. Nano Mater, 2(10), 6537–6545. DOI
- Ryder, C. R., Wood, J. D., Wells, S.A., Hersam, M. C. (2016) Chemicallytailoring semiconducting two-dimensional transition metal dichalcogenides andblack phosphorus. ACS Nano, 10, 3900–3917. DOI
- Wen, W., Song, Y., Yan, X., Zhu, C., Du, D., Wang, S., Asiri, A. M., Lin,Y. (2018) Recent advances in emerging 2D nanomaterials for biosensingand bioimaging applications. Mater. Today, 21, 164–177. DOI
- Xu, B. Z., Zhu, M. S., Zhang, W. C., Zhen, X., Pei, Z. X., Xue, Q., Zhi, C.Y., Shi, P. (2016) Ultrathin MXene-Micropattern-Based Field-Effect Transistorfor Probing Neural Activity. Adv. Mater, 28, 3333–3339. DOI
- Ge, Q., Li, C, Fan, Z., Xia, B., Zang, C., Chen, L., Zhao, C., Sang, H.,Wang, A. (2024) Nanoflower-shaped MXene-based field-effect transistorcapable of ultrasensitive microRNA-21 determination towards efficient lungcancer diagnosis. New J. Chem., 48, 9474–9479. DOI
- Qiao, Q., Wang, J., Li, B. (2024) Ti3C2Tx MXene nanosheet-baseddrug delivery/cascaded enzyme system for combination cancer therapyand anti-inflammation. Appl. Mater. Today, 38, 102215. DOI
- Qu, L., Wu, M., Zhao, L. (2023) A sandwich electrochemical immunosensorbased on MXene@dual MOFs for detection of tumor marker CA125.Microchimica Acta, 190, 147. DOI
- Majd, S. M., Salimi, A., Ghasemi, F. (2018) An ultrasensitive detectionof miRNA-155 in breast cancer via direct hybridization assay using twodimensional molybdenum disulfide field-effect transistor biosensor. Biosens.Bioelectron., 105, 6–13. DOI
- Lin, S., Chen, Y., Li, H., Wang, W., Wang, Y., Wu, M. (2024) Applicationof metal-organic frameworks and their derivates for thermal-catalyticC1 molecules conversion. iScience, 27(5), 109656. DOI
- Wang, Y., Sun, J., Tsubaki, N. (2023) Clever nanomaterials fabricationtechniques encounter sustainable C1 catalysis. Acc. Chem. Res, 56, 2341–2353. DOI
- Kreno, L. E., Leong, K., Farha, O. K., Allendorf, M., Van Duyne, R. P.,Hupp, J. T. (2012) Metal-organic framework materials as chemical sensors.Chem. Rev., 112, 1105–1125. DOI
- Yao, M. S., Lv, X.J., Fu, Z. H., Li, W. H., Deng, W. H., Wu, G. D., Xu, G.(2017) Layer-by-Layer Assembled Conductive Metal-Organic FrameworkNanofilms for Room-Temperature Chemiresistive Sensing. Angew. Chem., 56,16510–16514. DOI
- Barr, M. K. S., Nadiri, S., Chen, D. H., Weidler, P. G., Bochmann,S., Baumgart, H., Bachmann, J., Redel, E. (2022) Solution Atomic LayerDeposition of Smooth, Continuous, Crystalline Metal-Organic Framework ThinFilms. Chem. Mater., 34(22), 9836-9843. DOI
- Guo, L., Yang, L., Li, M., Kuang, L., Song, Y., Wang, L. (2021) Covalentorganic frameworks for fluorescent sensing: Recent developments and futurechallenges. Coord. Chem. Rev., 440, 213957. DOI
- Li, S. M., Zou, J., Tan, L. F., Huang, Z. B., Liang, P., Meng, X. W. (2022)Covalent organic frameworks: From linkages to biomedical applications. Chem.Eng. J., 446, 137148. DOI
- Shi, Y. Q., Yang, J. L., Gao, F., Zhang, Q. C. (2023) Covalent prganicframeworks: Recent progress in biomedical applications. ACS Nano, 17,1879–1905. DOI
- Akyuz, L. (2020) An imine based COF as a smart carrier for targeted drugdelivery: From synthesis to computational studies. Microporous MesoporousMater, 294, 109850. DOI
- Scicluna, M. C. Vella-Zarb, L. (2020) Evolution of nanocarrier drugdeliverysystems and recent advancements in covalent organic framework–drugsystems. ACS Appl. Nano Mater, 3, 3097–3115. DOI
- Jin, M., Zhao, Y. Y., Guan, Z. J., Fang, Y. (2023) Porous FrameworkMaterials for Bioimaging and Cancer Therapy. Molecules, 28, 1360. DOI
- Ma, J. X., T. Shu, T., Sun, Y. P., Zhou, X., Ren, C. Y., Su, L., Zhang, X.J. (2022) Luminescent Covalent Organic Frameworks for Biosensing andBioimaging Applications. Small, 18, 2103516. DOI
- Bagheri, A. R., Li, C. J., Zhang, X. L., Zhou, X. X., Aramesh, N., Zhou,H. Y., Jia, J. B. (2021) Recent advances in covalent organic frameworks forcancer diagnosis and therapy. Biomater. Sci., 9, 5745–5761. DOI
- Das, S. K., Roy, S., Das, A., Chowdhury, A., Chatterjee, N., Bhaumik, A.(2022) A conjugated 2D covalent organic framework as a drug delivery vehicletowards triple negative breast cancer malignancy. Nanoscale Adv., 4, 2313–2320. DOI
- Yue, Y., Ji, D., Liu, Y., Wei, D. (2024) Chemical Sensors Based onCovalent Organic Frameworks. Chemistry, 30(3), e202302474. DOI
- Cote, A. P., Benin, A. I., Ockwig, N. W., O’Keeffe, M., Matzger, A. J., Yaghi,O. M. (2005) Porous, crystalline, covalent organic frameworks. Science, 310,1166–1170. DOI
- Bhunia, S., Deo, K. A., Gaharwar, A. K. (2020) 2D Covalent OrganicFrameworks for Biomedical Applications. Adv. Funct. Mater., 30, 2002046. DOI
- Esrafili, A., Wagner, A., Inamdar, S., Acharya, A. P. (2021) CovalentOrganic Frameworks for Biomedical Applications. Adv. Healthcare Mater., 10,2002090. DOI
- Lohse, M. S., Bein, T. (2018) Covalent Organic Frameworks: Structures,Synthesis, and Applications. Adv. Funct. Mater., 28, 1705553. DOI
- Vardhan, H., Rummer, G., Deng, A., Ma, S. (2023) Large-Scale Synthesisof Covalent Organic Frameworks: Challenges and Opportunities. Membranes(Basel), 13(8), 696. DOI
- Zhao, X., Pachfule, P., Thomas, A. (2021) Covalent organic frameworks(COFs) for electrochemical applications. (Review Article) Chem. Soc. Rev., 50,6871-6913. DOI
- Côté, A. P., El-Kaderi, H. M., Furukawa, H., Hunt, J. R., Yaghi, O. M.(2007) Reticular synthesis of microporous and mesoporous 2D covalent organicframeworks. J. Am. Chem. Soc., 129, 12914–12915. DOI
- Lu, Z., Xu, K., Xiao, K. et al. (2025) Biomolecule sensors based on organicelectrochemical transistors. npj Flex Electron, 9, 9. DOI
- Thomas, S. A., Bekhti-Sari, F., Whelan, J., Alkhalifah, M. A., Khair,M., Traboulsi, H., Pasricha, R., Jagannathan, R., Mokhtari-Soulimane, N.,Gándara, F., Trabolsi, A., Benyettou, F., Kaddour, N., Prakasam, T., Das,G., Sharma, S. K. (2021) In vivo oral insulin delivery via covalent organicframeworks. Chem. Sci., 12(17), 6037-6047. DOI
- Uribe-Romo, F. J., Doonan, C. J., Furukawa, H., Oisaki, K., Yaghi, O. M.(2011) Crystalline covalent organic frameworks with hydrazone linkages. J.Am. Chem. Soc., 133, 11478–11481. DOI
- Das, G., Balaji, Shinde, D., Kandambeth, S., Biswal, B. P., Banerjee, R.(2014) Mechanosynthesis of imine, β-ketoenamine, and hydrogen-bondedimine-linked covalent organic frameworks using liquid-assisted grinding. Chem.Commun., 50(84), 12615–12618. DOI
- Puthiaraj, P., Lee, Y.-R., Zhang, S., Ahn, W.-S. (2016) Triazine-basedcovalent organic polymers: design, synthesis and applications in heterogeneouscatalysis. J. Mater. Chem. A, 4, 16288. DOI
- Ren, S., Bojdys, M. J., Dawson, R., Laybourn, A., Khimyak, Y. Z., Adams,D. J., Cooper, A. I. (2012) Porous, Fluorescent, Covalent Triazine-BasedFrameworks Via Room-Temperature and Microwave-Assisted Synthesis. Adv.Mater., 24(17), 2357– 2361. DOI
- Vitaku, E., Dichtel, W. R. (2017) Synthesis of 2D Imine-Linked CovalentOrganic Frameworks through Formal Transimination Reactions. J. Am. Chem.Soc., 139(37), 12911– 12914. DOI
- Ge, J., Xiao, J., Liu, L., Qiu, L., Jiang, X. (2016) Facile microwave-assistedproduction of Fe3O4 decorated porous melamine-based covalent organicframework for highly selective removal of Hg2+. J. Porous. Mater., 23(3),791–800. DOI
- Li, Z., Wang, W., Ndahiro, C., Zhou, X., Shen, S., Zhang, G. (2025) WS2quantum dots embedded CTF/PVDF membranes for efficient remediation ofdye wastewater with enhanced self-cleaning properties. J. Water Process Eng.,74, 107896. DOI
- Wang, Q. K., Ai, Z. L., Guo, Q. Y., Wang, X. J., Dai, C. H., et al. (2023)Photo-Enhanced Chemo-Transistor Platform for Ultrasensitive Assay of SmallMolecules. J. Am. Chem. Soc., 145, 10035–10044. DOI
- Anderson, N. L., Anderson, N. G. (2002) The human plasma proteome:history, character, and diagnostic prospects. Mol. Cell. Proteomics, 1(11), 845-867. DOI
- Yan, F., Zhang, M., Li, J. (2014) Solution–gated graphene transistorsfor chemical and biological sensors. Adv. Healthc. Mater., 3, 313–331. DOI
- Ju, P., Zhu, Y.-Y., Jiang, T.-T., Gao, G., Wang, S.-L., et al. (2023) DNAintercalation makes possible superior-gain organic photoelectrochemicaltransistor detection. Biosens. Bioelectron., 237, 5543. DOI
- Deng, M., Ren, Z., Zhang, H., Li, Z., Xue, C. et al. (2023) Unamplifiedand realtime label free miRNA21 detection using solution gated graphenetransistors in prostate cancer diagnosis. Adv. Sci., 10, 2205886. DOI
- Ma, X. et al. (2022) OFET and OECT, two types of organic thin-filmtransistor used in glucose and DNA biosensors: a review. IEEE Sens. J., 22,11405–11414. DOI
- Hou, L. et al. (2024) Reticular heterojunction for organicphotoelectrochemical transistor detection of neuron specific enolase. Small, 20,240003. DOI
- Ding, L. et al. (2022) Turning on high-sensitive organic electrochemicaltransistor based photoelectrochemical type sensor over modulation of Fe MOFby PEDOT. Adv. Funct. Mater., 32, 2202735. DOI
- Cai, H. et al. (2024) Molecule engineering metal–organic frameworkbasedorganic photoelectrochemical transistor sensor for ultrasensitive bilirubindetection. Anal. Chem., 96, 12739–12747. DOI
- Li, H., Fan, R., Zou, B, Yan, J., Shi, Q., Guo, G. (2023) Roles ofMXenes in biomedical applications: recent developments and prospects. J.Nanobiotechnology, 21, 73. DOI
- Xu, M., Chen, K., Zhu, L., Zhang, S., Wang, M., He, L., Zhang,Z., Du, M. (2021) MOF@COF Heterostructure Hybrid for Dual-ModePhotoelectrochemical−Electrochemical HIV-1 DNA Sensing. Langmuir., 37,13479–13492. DOI
- Li, Y., Zhang, C., He, Y., Gao, J., Li, W., Cheng, L., Sun, F., Xia, P., Wang,Q. A. (2022) Generic and Non-Enzymatic Electrochemical Biosensor IntegratedMolecular Beacon-like Catalyzed Hairpin Assembly Circuit with MOF@Au@G-Triplex/Hemin Nanozyme for Ultrasensitive Detection of miR-721.Biosens. Bioelectron., 203, 114051. DOI
- Liu, Y., Nie, Y., Wang, M., Zhang, Q., Ma, Q. (2020) Distance-Dependent Plasmon-Enhanced Electrochemiluminescence Biosensor Basedon MoS2 Nanosheets. Biosens. Bioelectron., 148, 111823. DOI
- Zhang, C., Shi, D., Li, X., Yuan, J. (2022) Microfluidic ElectrochemicalMagnetoimmunosensor for Ultrasensitive Detection of Interleukin-6 Basedon Hybrid of AuNPs and Graphene. Talanta, 240, 123173. DOI
- Guo, Y.-Z., Liu, J.-L., Chen, Y.-F., Chai, Y.-Q., Li, Z.-H., Yuan, R.(2022) Boron and Nitrogen-Codoped Carbon Dots as Highly EfficientElectrochemiluminescence Emitters for Ultrasensitive Detection of Hepatitis BVirus DNA. Anal. Chem., 94, 7601–7608. DOI
- Lu, Q., Su, T., Shang, Z., Jin, D., Shu, Y., Xu, Q., Hu, X. (2021) FlexiblePaper-Based Ni-MOF Composite/AuNPs/CNTs Film Electrode for HIVDNA Detection. Biosens. Bioelectron., 184, 113229. DOI
- Huang, S., Liu, Z., Yan, Y., Chen, J., Yang, R., Huang, Q., Jin, M., Shui, L.(2022) Triple signal-enhancing electrochemical aptasensor based on rhomboiddodecahedra carbonized-ZIF67 for ultrasensitive CRP detection. BiosensBioelectron., 207, 114129. DOI
- Xu, B. Z., Zhu, M. S., Zhang, W. C., Zhen, X., Pei, Z. X., Xue, Q., Zhi, C.Y., Shi, P. (2016) Ultrathin MXene-Micropattern-Based Field-Effect Transistorfor Probing Neural Activity. Adv. Mater., 28, 3333–3339. DOI
- Palanisamy, S. et al. (2023) One-step-one-pot hydrothermally derivedmetal-organic-framework-nanohybrids for integrated point-of-care diagnosticsof SARS-CoV-2 viral antigen/pseudovirus utilizing electrochemical biosensorchip. Sens. Actuators B, 390, 133960. DOI
- Dezhakam, E., Vayghan, R. F., Dehghani, S., Kafili-Hajlari, T., Naseri, A.,Dadashpour, M., Khalilzadeh, B., Kanberoglu, G. S. (2024) Highly efficientelectrochemical biosensing platform in breast cancer detection based on MOFCOF@Au core-shell like nanostructure. Sci. Rep., 14, 29850. DOI
- Chen, Z., Wu, C., Yuan, Y., Xie, Z., Li, T., Huang, H., Li, S., Deng, J., Lin,H., Shi, Z., et al. (2023) CRISPR-Cas13a-Powered Electrochemical Biosensorfor the Detection of the L452R Mutation in Clinical Samples of SARS-CoV-2Variants. J. Nanobiotechnol., 21, 141. DOI
- Cai, Q., Wu, D., Li, H., Jie, G., Zhou, H. (2021) VersatilePhotoelectrochemical and Electrochemiluminescence Biosensor Based on 3DCdSe QDs-DNA Nanonetwork-SnO2 Nanoflower Coupled with DNA WalkerAmplification for HIV Detection. Biosens. Bioelectron., 191, 113455. DOI
- Biswas, S., Lan, Q., Xie, Y., Sun, X., Wang, Y. (2021) Label-FreeElectrochemical Immunosensor for Ultrasensitive Detection of CarbohydrateAntigen 125 Based on Antibody-Immobilized Biocompatible MOF-808/CNT.ACS Appl. Mater. Interfaces, 13, 3295–3302. DOI
- Chen, G. et al. (2023) High-efficiency aluminum-metal organicframework/HEPES electrochemiluminescence system for ultrasensitivedetection of HBV DNA. Anal. Chem., 95, 7030–7035. DOI
- An, Y., Dong, S., Chen, H., Guan, L., Huang, T. (2022) Ce-MOF/COF/carbon nanotube hybrid composite: Construction of efficient electrochemicalimmune platform for amplifying detection performance of CA125.Bioelectrochemistry, 147, 108201. DOI
- Sobhanie, E., Salehnia, F., Xu, G., Hamidipanah, Y., Arshian, S.,Firoozbakhtian, A., Hosseini, M., Ganjali, M. R., Hanif, S. (2022) Recent Trendsand Advancements in Electrochemiluminescence Biosensors for Human VirusDetection. Trends Anal. Chem., 157, 116727. DOI
- Zhang, Y.-W., Liu, W.-S., Chen, J.-S., Niu, H.-L., Mao, C.-J., Jin, B.-K.(2020) Metal-organic gel and metal-organic framework based switchableelectrochemiluminescence RNA sensing platform for Zika virus. Sensor.Actuator. B Chem., 321, DOI
- Zhang, H.-J., Zhu, J., Bao, N., Ding, S.-N. (2021) Enhancedelectrochemiluminescence of CdS quantum dots capped with mercaptopropionicacid activated by EDC for Zika virus detection. Analyst, 146(9), 2928–2935. DOI
- Yang, T., Xu, C., Liu, C., Ye, Y., Sun, Z., Wang, B., Luo, Z. (2022)Conductive Polymer Hydrogels Crosslinked by Electrostatic Interaction withPEDOT:PSS Dopant for Bioelectronics Application. Chem. Eng. J., 429,132430. DOI
- Luo, G. (2019) Electrochemical Myoglobin Biosensor Based onMagnesium Metal-Organic Frameworks and Gold Nanoparticles CompositeModified Electrode. Int. J. Electrochem. Sci., 14, 2405–2413. DOI
- Li, S., Hu, C., Chen, C., Zhang, J., Bai, Y., Tan, C. S., Ni, G., He, F., Li,W., Ming, D. (2021) Molybdenum Disulfide Supported on Metal-OrganicFrameworks as an Ultrasensitive Layer for the Electrochemical Detection of theOvarian Cancer Biomarker CA125. ACS Appl. Bio Mater., 4, 5494–5502. DOI
- Xue, Y., Wang, Y., Feng, S., Yan, M., Huang, J., Yang, X. (2022) A Dual-Amplification Mode and Cu-Based Metal-Organic Frameworks MediatedElectrochemical Biosensor for Sensitive Detection of MicroRNA. Biosens.Bioelectron., 202, 113992. DOI
- Lu, J., Hu, Y., Wang, P., Liu, P., Chen, Z., Sun, D. (2020) ElectrochemicalBiosensor Based on Gold Nanoflowers-Encapsulated Magnetic Metal-OrganicFramework Nanozymes for Drug Evaluation with in-Situ Monitoring of H2O2Released from H9C2 Cardiac Cells. Sens. Actuators B, 311, 127909. DOI
- Du, L., Chen, W., Wang, J., Cai, W., Kong, S., Wu, C. (2019) Folic Acid-Functionalized Zirconium Metal-Organic Frameworks Based ElectrochemicalImpedance Biosensor for the Cancer Cell Detection. Sens. Actuators B, 301,127073. DOI
- Chang, J., Wang, X., Wang, J., Li, H., Li, F. (2019) Nucleic Acid-Functionalized Metal-Organic Framework-Based HomogeneousElectrochemical Biosensor for Simultaneous Detection of Multiple TumorBiomarkers. Anal. Chem., 91, 3604–3610. DOI
- Wang, L., Meng, T., Liang, L., Sun, J., Wu, S., Wang, H., Yang, X., Zhang,Y. (2019) Fabrication of Amine-Functionalized Metal-Organic Frameworkswith Embedded Palladium Nanoparticles for Highly Sensitive ElectrochemicalDetection of Telomerase Activity. Sens. Actuators B, 278, 133–139. DOI
- Huang, S., Lu, M., Wang, L. (2020) Cytochrome C-MultiwalledCarbon Nanotube and Cobalt Metal Organic Framework/Gold NanoparticleImmobilized Electrochemical Biosensor for Nitrite Detection. RSC Adv., 11,501–509. DOI
- Gupta, A., Bhardwaj, S. K., Sharma, A. L., Kim, K. H., Deep A. (2019)Development of an Advanced Electrochemical Biosensing Platform for E. coliUsing Hybrid Metal-Organic Framework/Polyaniline Composite. Environ. Res.,171, 395–402. DOI
- Yildirim, O., Derkus, B. (2020) Triazine-Based, 2D Covalent OrganicFrameworks Improve the Electrochemical Performance of EnzymaticBiosensors. J. Mater. Sci., 55, 3034–3044. DOI
- Xiao, Y., Wu, N., Wang, L., Chen, L. (2022) A Novel Paper-BasedElectrochemical Biosensor Based on N, O-Rich Covalent Organic Frameworksfor Carbaryl Detection. Biosensors, 12, 899. DOI
- Sun, X., Xie, Y., Chu, H., Long, M., Zhang, M., Wang, Y., Hu, X. (2022) AHighly Sensitive Electrochemical Biosensor for the Detection of HydroquinoneBased on a Magnetic Covalent Organic Framework and Enzyme for SignalAmplification. New J. Chem., 46, 11902–11909. DOI
- Li, H., Kou, B., Yuan, Y., Chai, Y., Yuan, R. (2022) Porous Fe3O4@COF-Immobilized Gold Nanoparticles with Excellent Catalytic Performancefor Sensitive Electrochemical Detection of ATP. Biosens. Bioelectron., 197,113758. DOI
- Han, Y., Lu, J., Wang, M., Sun, C., Yang, J., Li, G. (2022) AnElectrochemical Biosensor for Exosome Detection Based on Covalent OrganicFrameworks Conjugated with DNA and Horseradish Peroxidase. J. Electroanal.Chem., 920, 116576. DOI
- Liang, W., Carraro, F., Solomon, M. B., Bell, S. G., Amenitsch, H., et al.(2019) Enzyme encapsulation in a porous hydrogen-bonded organic framework.J. Am. Chem. Soc., 141, 36. DOI
- Li, P., He, Y., Guang, J., Weng, L., Zhao, J. C. G., et al. (2014) Ahomochiral microporous hydrogen-bonded organic framework for highlyenantioselective separation of secondary alcohols. J. Am. Chem. Soc., 136, 547. DOI
- Li, P., He, Y., Zhao, Y., Weng, L., Wang, H., et al. (2015) A rod-packingmicroporous hydrogen-bonded organic framework for highly selectiveseparation of C2H2/CO2 at room temperature. Angew. Chem. Int. Ed., 54, 574. DOI
- Wang, H., Li, B., Wu, H., Hu, T. L., Yao, Z. et al. (2015) A flexiblemicroporous hydrogen-bonded organic framework for gas sorption andseparation. J. Am. Chem. Soc., 137, 9963. DOI
- Yang, W., Yang, F., Hu, T. L., King, S. C., Wang, H. et al. (2016)Microporous diaminotriazine-decorated porphyrin-based hydrogen-bondedorganic framework: permanent porosity and proton conduction. Cryst. GrowthDes., 16, 5831. DOI
- Yuan, S., Zou, L., Qin, J. S., Li, J., Huang, L. et al. (2017) Constructionof hierarchically porous metal–organic frameworks through linker labilization.Nat. Commun., 8, 15356. DOI
- Wang, H., Bao, Z., Wu, H., Lin, R. B., Zhou, W. et al. (2017) Two solventinducedporous hydrogen-bonded organic frameworks: solvent effects onstructures and functionalities. Chem. Commun., 53, 11150. DOI
- Liu, B. T., Pan, X. H., Nie, D.Y., Hu, X. J., Liu, E. P., et al. (2020) Ionichydrogen-bonded organic frameworks for ion-responsive antimicrobialmembranes. Adv. Mater., 32, 48. DOI
- Wied, P., Carraro, F., Bolivar, J. M., Doonan, C. J., Falcaro, P., et al.(2022) Combining a genetically engineered oxidase with hydrogen-bondedorganic frameworks (HOFs) for highly efficient biocomposites. Angew. Chem.Int. Ed., 61, 16. doi: 10.1002/anie.202117345
- Yin, Q., Zhao, P., Sa, R. J., Chen, G. C., Lü, J. Liu, T. F., Cao, R. (2018) Anultra-robust and crystalline redeemable hydrogen-bonded organic frameworkfor synergistic chemo-photodynamic therapy. Angew. Chem. Int. Ed., 57,7691–7696. DOI
- Li, J., Chen, B. (2024) Flexible hydrogen-bonded organic frameworks(HOFs): opportunities and challenges. Chem. Sci., 15(26), 9874-9892. DOI
- Mohan, B., Singh, G., Gupta, R. K., Sharma P. K., Solovev A. A., PombeiroA. J. L., Ren P. (20245) Hydrogen-bonded organic frameworks (HOFs):Multifunctional material on analytical monitoring. TrAC Trends. Anal. Chem.,170, 117436. DOI
- Jiang, R., Luo, G., Chen, G., Lin, Y., Tong, L., Huang, A., Zheng, Y., Shen,Y., Huang, S., Ouyang G. (2024) Boosting the photocatalytic decontaminationefficiency using a supramolecular photoenzyme ensemble. Sci. Adv., 10(37),eadp1796. DOI
- Vijayakanth, T., Dasgupta, S., Ganatra, P., Rencus-Lazar, S., Desai, A. V.,Nandi, S., Jain, R., Bera, S., Nguyen, A. I., Gazit, E., Misra, R. (2024) Peptidehydrogen-bonded organic frameworks. Chem. Soc. Rev., 53(8), 3640-3655. DOI
- Chafiq, M., Chaouiki, A., Ko, Y. G. (2023) Recent Advances inMultifunctional Reticular Framework Nanoparticles: A Paradigm Shift inMaterials Science Road to a Structured Future. Nanomicro Lett., 15(1), 213. DOI
- Zhang, H.-Y., Yang, Y., Li, C.-C., Tang, H.-L., Zhang, F.-M., Zhang,G.-L., Yan, H. (2021) A new strategy for constructing covalently connectedMOF@ COF core–shell heterostructures for enhanced photocatalytic hydrogenevolution. J. Mater. Chem. A, 9, 16743. DOI
- Peng, Y., Zhao, M., Chen, B., Zhang, Z., Huang, Y., et al. (2018)Hybridization of MOFs and COFs: a new strategy for construction of MOF@COF core–shell hybrid materials. Adv. Mater., 30, 1705454. DOI
- Altintas, C., Erucar, I., Keskin, S. (2022) MOF/COF hybrids as nextgeneration materials for energy and biomedical applications. CrystEngComm,24(42), 7360-7371. DOI
- Cui, B., Fu, G. (2022) Process of metal-organic framework (MOF)/covalent-organic framework (COF) hybrids-based derivatives and theirapplications on energy transfer and storage. Nanoscale, 14(5), 1679-1699. DOI
- Liang, H., Wang, L., Yang, Y., Song, Y., Wang, L. (2021) A novelbiosensor based on multienzyme microcapsules constructed from covalentorganicframework. Biosens. Bioelectron., 193, 113553. DOI
- Hota, M. K. et al. (2022) Electrochemical thin-film transistors usingcovalent organic framework channel. Adv. Funct. Mater., 32, 2201120. DOI
- Daniel M., Mathew G., Anpo M., Neppolian B. (2022) MOF basedelectrochemical sensors for the detection of physiologically relevantbiomolecules: an overview. Coord. Chem. Rev., 468, 214627. DOI
- Wang, Z., Shi, X., Chen, F., Fan, G., Zhao, W. (2024) Ag/AgCl likephotogating of a COF on MOF heterojunction in organic photoelectrochemicaltransistor. Adv. Funct. Mater., 34, 2404497. DOI
- Lu, Z., Xu, K., Xiao, K. et al. (2025) Biomolecule sensors based on organicelectrochemical transistors. npj Flex Electron, 9, 9. DOI.org/10.1038/s41528-025-00383-x
- Sakata T. (2024) Signal transduction interfaces for field-effect transistorbasedbiosensors. Commun. Chem., 7, 35. DOI
- Deng, M., Li, J., Xiao, B., Ren, Z., Li, Z., Yu, H., Li, J., Wang, J., Chen, Z.,Wang, X. (2022) Ultrasensitive Label-Free DNA Detection Based on Solution-Gated Graphene Transistors Functionalized with Carbon Quantum Dots. Anal.Chem., 94, 3320. DOI
- Xu, M., Chen, K., Zhu, L., Zhang, S., Wang, M., He, L., Zhang,Z., Du, M. (2021) MOF@COF Heterostructure Hybrid for Dual-ModePhotoelectrochemical-Electrochemical HIV-1 DNA Sensing. Langmuir, 37,13479-13492. DOI
- Gao G. (2022) Hybridization chain reaction for regulating surfacecapacitance of organic photoelectrochemical transistor toward sensitive miRNAdetection. Biosens. Bioelectron., 209, 114224. DOI
- Dezhakam, E., Vayghan, R. F., Dehghani, S. et al. (2024) Highly efficientelectrochemical biosensing platform in breast cancer detection based on MOFCOF@Au core-shell like nanostructure. Sci. Rep., 14, 29850. DOI
- Fu, J., Das, S., Xing, G., Ben, T., Valtchev, V., Qiu, S. (2016) Fabricationof COF-MOF Composite Membranes and Their Highly Selective Separation ofH2/CO2. J. Am. Chem. Soc., 138, 7673-7680. DOI
- Zhou, N., Ma, Y., Hu, B., He, L., Wang, S., Zhang, Z., Lu, S. (2019)Construction of Ce-MOF@COF hybrid nanostructure: Label-free aptasensorfor the ultrasensitive detection of oxytetracycline residues in aqueoussolution environments. Biosens. Bioelectron., 127, 92-100. DOI
- Nakatani, R., Irie, T., Das, S., Fang, Q., Negish, Y. (2025) Converging theComplementary Traits of Metal–Organic Frameworks and Covalent OrganicFrameworks. ACS Appl. Mater. Interfaces, 17(17), 24701-24729. DOI
- Mondal, T., Haldar, D., Ghosh, A., Ghorai, U. K., Saha, S. K. (2020) AMOF functionalized with CdTe quantum dots as an efficient white light emittingphosphor material for applications in displays. New J. Chem., 44, 55–63. DOI
- Yang, Q., Wang, Q., Long, Y., Wang, F., Wu, L., Pan, J., et al. (2020) Insitu formation of Co9S8 quantum dots in MOF-derived ternary metal layereddouble hydroxide nanoarrays for high-performance hybrid supercapacitors. Adv.Energy. Mater., 10, 1903193. DOI
- Gui, B., Meng, Y., Xie, Y., Tian, J., Yu, G., Zeng, W., Zhang, G., Gong,S., Yang, C., Zhang, D., Wang, C. (2018) Tuning the Photoinduced ElectronTransfer in a Zr-MOF: Toward Solid-State Fluorescent Molecular Switch andTurn-On Sensor. Adv. Mater., 30(34), 1802329. DOI
- Huang, Z., Chen, H., Zhao, L., He, X., Fang, W., Du, Y., et al. (2018) CdSeQDs sensitized MIL-125/TiO2@SiO2 biogenic hierarchical composites withenhanced photocatalytic properties via two-level heterostructure. J. Mater. Sci.Mater. Electron., 29, 1245-12054. DOI
- Jin, M., Mou, Z. L., Zhang, R.-L., Liang, S. S., Zhang, Z. Q. (2017) Anefficient ratiometric fluorescence sensor based on metal-organic frameworksand quantum dots for highly selective detection of 6-mercaptopurine. Biosens.Bioelectron., 91, 162–168. DOI
- Li, Z., Bu, F., Wei, J., Yao, W., Wang, L., Chen, Z., et al. (2018) Boostingthe energy storage densities of supercapacitors by incorporating N-dopedgraphene quantum dots into cubic porous carbon. Nanoscale, 10, 22871–22883. DOI
- Li, Z., Liu, X., Wang, L., Bu, F., Wei, J., Pan, D., et al. (2018) Hierarchical3D all-carbon composite structure modified with N-doped graphene quantumdots for high-performance flexible supercapacitors. Small, 14, 1801498. DOI
- Sun Y., Zheng L., Yang Y. et al. (2020) Metal–Organic FrameworkNanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett.,12, 103. DOI
- Giri, L., Rout, S. R., Varma, R. S., Otyepka, M., Jayaramulu, K., Dandela,R. (2022) Recent advancements in metal–organic frameworks integratingquantum dots (QDs@MOF) and their potential applications. ScienceOpen, Inc.Nanotechnol. Rev., 11(1), 1947-1976. DOI
- Li, K., Ji, Q., Liang, H., Hua, Z., Hang, X., Zeng, L., Han, H.(2023) Biomedical application of 2D nanomaterials in neuroscience. J.Nanobiotechnology, 21, 181. DOI
- Wang, M., Nian, L., Cheng, Y., Yuan, B., Cheng, S., Cao, C. (2021)Encapsulation of Colloidal Semiconductor Quantum Dots into Metal-OrganicFrameworks for Enhanced Antibacterial Activity through Interfacial ElectronTransfer. Chem. Eng. J., 426 (5), 130832. DOI
- Li R., Qu X.-L., Zhang Y.-H., Han H.-L., Li X. (2016) Lanthanide–organicframeworks constructed from naphthalenedisulfonates: structure, luminescenceand luminescence sensing properties. CrystEngComm, 18, 5890. DOI
- Dong, J., Zhao, D., Lu, Y., Sun, W.-Y. (2019) Photoluminescent metal–organic frameworks and their application for sensing biomolecules. J. Mater.Chem. A, 7, 22744-22767. DOI
- Picchi, D. F., Biglione, C., Horcajada, P. (2023) Nanocomposites Basedon Magnetic Nanoparticles and Metal-Organic Frameworks for Therapy,Diagnosis, and Theragnostics. ACS Nanosci. Au., 4(2), 85-114. DOI
- Abdelhamid, H. N. (2021) Zeolitic Imidazolate Frameworks (ZIF-8) forBiomedical Applications: A Review. Curr. Med. Chem., 28(34), 7023-7075. DOI
- Hoseinpour, V., Shariatinia, Z. (2021) Applications of zeolitic imidazolateframework-8 (ZIF-8) in bone tissue engineering: A review. Tissue Cell, 72,101588. DOI
- Gatou, M. A., Vagena, I. A., Lagopati, N., Pippa, N., Gazouli, M.,Pavlatou, E. A. (2023) Functional MOF-Based Materials for Environmental andBiomedical Applications: A Critical Review. Nanomaterials (Basel), 13(15),2224. DOI
- Narea, P., Brito, I., Quintero, Y., Camú, E. (2023) Novel HydrophobicFunctionalized UiO-66 Series: Synthesis, Characterization, and Evaluation ofTheir Structural and Physical-Chemical Properties. Int. J. Mol. Sci., 25(1), 199. DOI
- Yang, P., Liu, Q., Liu, J., Zhang, H., Li, Z., Li, R., et al. (2017) Interfacialgrowth of a metal-organic framework (UiO-66) on functionalized grapheneoxide (GO) as a suitable seawater adsorbent for extraction of uranium(vi). J.Mater. Chem. A, 5, 17933–17942. DOI
- Yang, C., Shang, S., Gu, Q., Shang, J., Li, X. (2022) Metal-organicframework-derived carbon nanotubes with multi-active Fe-N/Fe sites as abifunctional electrocatalyst for zinc-air battery. J. Energy. Chem., 66, 306–313. DOI
- Wu, L. Y., Mu, Y. F., Guo, X. X., et al. (2019) Encapsulating PerovskiteQuantum Dots in Iron-Based Metal–Organic Frameworks (MOFs) for EfficientPhotocatalytic CO2 Reduction. Angew. Chem. Int. Ed., 58, 9491-9495. DOI
- Zhang, D., Zhao, J., Liu, Q., Xia, Z. (2019) Synthesis and luminescenceproperties of CsPbX3@Uio-67 composites toward stable photoluminescenceconvertors. Inorg. Chem., 58, 1690–1696. DOI
- Meng, X., Zhang, C., Zhuang, J., Zheng, G., Zhou, L. (2019) Metal-organicframework as nanoreactors to co-incorporate carbon nanodots and CdS quantumdots into the pores for improved H2 evolution without noble-metal cocatalyst.Appl. Catal. B. Environ., 244, 340–346. DOI
- Ren, J., Li, T., Zhou, X., Dong, X., Shorokhov, A. V., Semenov, M. B.,et al. (2019) Encapsulating all-inorganic perovskite quantum dots intomesoporous metal organic frameworks with significantly enhanced stabilityfor optoelectronic applications. Chem. Eng. J., 358, 30–39. DOI
- Mo, G., Qin, D., Jiang, X., Zheng, X., Mo, W., Deng, B. (2020) A sensitiveelectrochemiluminescence biosensor based on metal-organic frameworkand imprinted polymer for squamous cell carcinoma antigen detection. SensActuators B Chem., 310, 127852. DOI
- Wang, K., Li, N., Zhang, J., Zhang, Z. (2017) Size-selective QD@MOFcore-shell nanocomposites for the highly sensitive monitoring of oxidaseactivities. Biosens. Bioelectron., 87, 339–344. DOI
- Wang, H., Yuan, X., Wu, Y., Chen, X., Leng, L., Zeng, G. (2015)Photodeposition of metal sulfides on titanium metal-organic frameworks forexcellent visible-light-driven photocatalytic Cr(vi) reduction. RSC Adv., 5,32531–32535. DOI. org/10.1039/C5RA01283J
- Lin, R., Shen, L., Ren, Z., Wu, W., Tan, Y., Fu, H., Zhang, J., Wu, L. (2014)Enhanced photocatalytic hydrogen production activity via dual modification ofMOF and reduced graphene oxide on CdS. Chem Commun (Camb), 50(62),8533-8535. DOI
- Murugesan, A., Li, H., Shoaib, M. (2025) Recent Advances inFunctionalized Carbon Quantum Dots Integrated with Metal-OrganicFrameworks: Emerging Platforms for Sensing and Food Safety Applications.Foods, 14(12), 2060. DOI
- Rabiee, N., Bagherzadeh, M., Jouyandeh, M., Zarrintaj, P., Saeb, M.R., Mozafari, M., Shokouhimehr, M., Varma, R. S. (2021) Natural PolymersDecorated MOF-MXene Nanocarriers for Co-delivery of Doxorubicin/pCRISPR. ACS Appl. Bio. Mater., 4(6), 5106-5121. DOI
- Chang, Y., Lou, J., Yang, L., Liu, M., Xia, N, Liu, L. (2022) Design andApplication of Electrochemical Sensors with Metal–Organic Frameworks as theElectrode Materials or Signal Tags. Nanomaterials. (Basel), 12(18), 3248. DOI
- Alsaiari, S. K., Qutub, S. S., Sun, S., Baslyman, W., Aldehaiman, M.,Alyami, M., Almalik, A., Halwani, R., Merzaban, J., Mao, Z., Khashab, N. M.(2021) Sustained and targeted delivery of checkpoint inhibitors by metalorganicframeworks for cancer immunotherapy. Sci. Adv., 7(4), eabe7174. DOI
- Kamal, N. A., Abdulmalek, E., Fakurazi, S., Cordova, K. E., AbdulRahman, M. B. (2022) Dissolution and Biological Assessment of Cancer-Targeting Nano-ZIF-8 in Zebrafish Embryos. ACS Biomater. Sci. Eng., 8(6),2445-2454. DOI
- Gu, C., Guo, C., Li, Z., Wang, M., Zhou, N., He, L., et al. (2019) BimetallicZrHf-based metal-organic framework embedded with carbon dots: ultrasensitiveplatform for early diagnosis of HER2 and HER2-overexpressed livingcancer cells. Biosens. Bioelectron., 134, 8–15. DOI
- Freitas, M., Nouws, H. P., Keating, E., Delerue-Matos, C. (2020) Highperformanceelectrochemical immunomagnetic assay for breast cancer analysis.Sens. Actuators B. Chem., 308, 127667. DOI
- Ehzari, H., Samimi, M., Safari, M., Gholivand, M. B. (2020) Label-freeelectrochemical immunosensor for sensitive HER2 biomarker detection usingthe core-shell magnetic metal-organic frameworks. J. Electroanalytical. Chem.,877, 114722. DOI
- Xie, H., Liu, X., Huang, Z., Xu, L., Bai, R., He, F., Wang, M., Han, L., Bao,Z., Wu, Y., Xie, C., Gong, Y. (2022) Nanoscale Zeolitic Imidazolate Framework(ZIF)-8 in Cancer Theranostics: Current Challenges and Prospects. Cancers(Basel), 14(16), 3935. DOI
- Smith, B. R., Cheng, Z., De, A., Rosenberg J., Gambhir S. S. (2010)Dynamic visualization of RGD-quantum dot binding to tumor neovasculatureand extravasation in multiple living mouse models using intravital microscopy.Small, 6(20), 2222-2229. doi: 10.1002/smll.201001022
- Kamal, N., Abdulmalek, E., Fakurazi, S., Cordova, K. E., AbdulRahman, M. B. (2021) Surface peptide functionalization of zeoliticimidazolate framework-8 for autonomous homing and enhanced delivery ofchemotherapeutic agent to lung tumor cells. Dalton Trans., 50(7), 2375-2386. DOI
- Sameni, M., Moradbeigi, P., Hosseini, S., Ghaderian, S. M. H., Jajarmi,V., Miladipour, A. H., Basati, H., Abbasi, M., Salehi, M. (2024) ZIF-8Nanoparticle: A Valuable Tool for Improving Gene Delivery in Sperm-MediatedGene Transfer. Biol. Proced. Online, 26(1), 4. DOI
- Barkalina, N., Charalambous, C., Jones, C., Coward, K. (2014)Nanotechnology in reproductive medicine: emerging applications ofnanomaterials. Nanomed. Nanotechnol. Biol. Med., 10(5), e921–938. DOI
- Acharya, B., Behera, A., Behera, S., Moharana, S. (2024) Recent Advancesin Nanotechnology-Based Drug Delivery Systems for the Diagnosis andTreatment of Reproductive Disorders. ACS Applied Bio Materials, 7(3), 1336-1361. DOI
- Gu, C., Guo, C., Li, Z., Wang, M., Zhou, N., He, L., et al. (2019) BimetallicZrHf-based metal-organic framework embedded with carbon dots: ultrasensitiveplatform for early diagnosis of HER2 and HER2-overexpressed livingcancer cells. Biosens. Bioelectron., 134, 8–15. DOI
- Ehzari H., Amiri M., Safari M. (2020) Enzyme-free sandwich-typeelectrochemical immunosensor for highly sensitive prostate specific antigenbased on conjugation of quantum dots and antibody on surface of modifiedglassy carbon electrode with core–shell magnetic metal-organic frameworks.Talanta, 210, 120641. DOI
- Zhang, Q., Tian, Y., Liang, Z., Wang, Z., Xu, S., Ma, Q. (2021)DNA-mediated Au–Au dimerbased surface plasmon couplingelectrochemiluminescence sensor for BRCA1 gene detection. Anal. Chem.,93(6), 3308–3314.
- Ehzari, H., Safari, M., Samimi, M. (2021) Signal Amplification of NovelSandwich-Type Genosensor via Catalytic Redox-Recycling on PlatformMWCNTs/Fe3O4@TMU-21 for BRCA1 Gene Detection. Talanta, 234, 122698. DOI
- Freitas, M., Nouws, H. P., Keating, E., Delerue-Matos, C. (2020) Highperformanceelectrochemical immunomagnetic assay for breast cancer analysis.Sens. Actuators B. Chem., 308, 127667. DOI
- Li, Z., Peng, Y., Xia, X. et al. (2019) Sr/PTA Metal Organic Framework asA Drug Delivery System for Osteoarthritis Treatment. Sci. Rep., 9, 17570. DOI
- Dou, M., Sanjay, S. T., Dominguez, D. C., Liu, P., Xu, F., Li, X. (2017)Multiplexed instrument-free meningitis diagnosis on a polymer/paper hybridmicrofluidic biochip. J. Biosens. Bioelectron., 87, 865–873. DOI
- Pan, Y., Zhan, S., Xia, F. (2018) Zeolitic imidazolate framework-basedbiosensor for detection of HIV-1 DNA. Anal. Biochem, 546, 5-9. DOI
- Qin, L., Lin, L.-X., Fang, Z.-P., Yang, S.-P., Qiu, G.-H., Chen, J.-X.,Chen, W.-H. (2016) A water-stable metal–organic framework of a zwitterioniccarboxylate with dysprosium: a sensing platform for Ebolavirus RNAsequences. Chem. Commun., 52(1), 132-135. DOI
- Yang, S. P., Chen, S. R., Liu, S. W., Tang, X. Y., Qin, L., Qiu, G. H., Chen,J. X., Chen, W. H. (2015) Platforms formed from a three-dimensional Cubasedzwitterionic metal-organic framework and probe ss-DNA: selectivefluorescent biosensors for human immunodeficiency virus 1 ds-DNA and sudanvirus RNA sequences. Anal Chem., 87(24), 12206–12214. DOI
- Xie, B. P., Qiu, G. H., Hu, P. P., Liang, Z., Liang, Y. M., Sun, B., Bai, L.P., Jiang, Z. H., Chen, J. X. (2018) Simultaneous detection of Dengue andZika virus RNA sequences with a three-dimensional Cu-based zwitterionicmetal–organic framework, comparison of single and synchronous fluorescenceanalysis. Sensors Actuators, B Chem., 254, 1133–1140.
- Xie, B. P., Qiu, G. H., Sun, B., Yang, Z. F., Zhang, W. H., Chen, J. X., Jiang,Z. H. (2019) Synchronous sensing of three conserved sequences of Zika virususing a DNAs@MOF hybrid: Experimental and molecular simulation studies.Inorg. Chem. Front., 6(1), 148–152. DOI
- Luo, L., Zhang, F., Chen, C., Cai, C. (2020) Molecular imprintingresonance light scattering nanoprobes based on pH-responsive metal-organicframework for determination of hepatitis A virus. Microchim. Acta, 187, 1–8. DOI
- Zhang, H. T., Zhang, J. W., Huang, G., Du, Z. Y., Jiang, H. L. (2014) Anamine-functionalized metal-organic framework as a sensing platform for DNAdetection. Chem. Commun., 50(81), 12069–12072. DOI
- Yang, J., Feng, W, Liang, K, Chen, C, Cai, C. (2020) A novel fluorescencemolecularly imprinted sensor for Japanese encephalitis virus detection based onmetal organic frameworks and passivation-enhanced selectivity. Talanta, 212,120744. DOI
- Quijia, C. R., Alves, R. C., Hanck-Silva, G., Frem, R. C. G., Arroyos,G., Chorilli, M. (2022) Metal-organic frameworks for diagnosis andtherapy of infectious diseases. Crit. Rev. Microbiol, 48(2), 161-196. DOI
- Cai, M., Ni, B., Hu, X., Wang, K. et al. (2022) An Investigation ofIRMOF-16 as a pH-responsive Drug Delivery Carrier of Curcumin. J. Sci. Adv.Mater. Devices, 7(4), 100507. DOI
- Chen, G., Luo, J., Cai, M., Qin, L., Wang, Y., Gao, L., Huang, P., Yu, Y.,Ding, Y., Dong, X., et al. (2019) Investigation of Metal-Organic Framework-5(MOF-5) as an Antitumor Drug Oridonin Sustained Release Carrier. Molecules,24, 3369. DOI
- Trushina, D. B., Sapach, A. Y., Burachevskaia, O. A., Medvedev, P. V.,Khmelenin, D. N., Borodina, T. N., Soldatov, M. A., Butova, V. V. (2022)Doxorubicin-Loaded Core-Shell UiO-66@SiO2 Metal-Organic Frameworks forTargeted Cellular Uptake and Cancer Treatment. Pharmaceutics, 14, 1325. DOI
- Safinejad, M., Rigi, A., Zeraati, M., Heidary, Z., Jahani, S., Chauhan, N.P. S., Sargazi, G. (2022) Lanthanum-based metal organic framework (La-MOF)use of 3,4-dihydroxycinnamic acid as drug delivery system linkers in humanbreast cancer therapy. BMC Chem., 16, 93. DOI
- Liu, Y., Zhang, H., Chen, T., Xu, C., Bao, X. (2024) Metal-organicframeworks (MOFs) and their derivatives as emerging biomaterials for thetreatment of osteoarthritis. Front. Pharmacol., 15, 1462368. DOI
- Gatou, M. A., Vagena, I. A., Lagopati, N., Pippa, N., Gazouli, M.,Pavlatou, E. A. (2023) Functional MOF-Based Materials for Environmental andBiomedical Applications: A Critical Review. Nanomaterials (Basel), 13(15),2224. DOI
- Al Sharabati, M., Sabouni, R., Husseini, G. A. (2022) BiomedicalApplications of Metal−Organic Frameworks for Disease Diagnosis andDrug Delivery: A Review. Nanomaterials (Basel), 12(2), 277. DOI
- Sadiq, S., Khan, S., Khan, I., Khan, A., Humayun, M., Wu, P., Usman, M.,Khan, A., Alanazi, A. F., Bououdina, M. (2024) A critical review on metalorganicframeworks (MOFs) based nanomaterials for biomedical applications:Designing, recent trends, challenges, and prospects. Heliyon, 10(3), e25521. DOI
- Gatou, M. A., Vagena, I. A., Lagopati, N., Pippa, N., Gazouli, M.,Pavlatou, E. A. (2023) Functional MOF-Based Materials for Environmental andBiomedical Applications: A Critical Review. Nanomaterials (Basel), 13(15),2224. DOI
- Lu, K., Aung, T., Guo, N., Weichselbaum, R., Lin, W. (2018) NanoscaleMetal-Organic Frameworks for Therapeutic, Imaging, and SensingApplications. Adv. Mater., 30(37), e1707634. DOI
- Suresh, K., Matzger, A. J. (2019) Enhanced Drug Delivery by Dissolutionof Amorphous Drug Encapsulated in a Water Unstable Metal–OrganicFramework (MOF) Angew. Chem. Int. Ed., 131, 16946–16950. DOI
- Zhong, Y., Liu, W., Rao, C., Li, B., Wang, X., Liu, D., Pan, Y., Liu, J. (2021)Recent advances in Fe-mof compositions for biomedical applications. Curr.Med. Chem., 28(30), 6179–6198. DOI
- Luo, Z., Fan, S., Gu, C., Liu, W., Chen, J., Li, B., Liu, J. (2019) Metal–organic framework (MOF)-based nanomaterials for biomedical applications.Curr. Med. Chem., 26(18), 3341–3369. DOI
- Abdelhamid, H. N. (2019) Surfactant assisted synthesis of hierarchicalporous metal-organic frameworks nanosheets. Nanotechnology, 30(43), 435601. DOI
- Lawson, H. D., Walton, S. P., Chan, C. (2021) Metal–organic frameworksfor drug delivery: a design perspective. ACS Appl. Mater. Interfaces, 13(6),7004–7020. DOI
- Abánades Lázaro, I., Wells, C. J., Forgan, R. S. (2020) Multivariatemodulation of the zr MOF UiO-66 for defect‐controlled combinationanticancer drug delivery. Angew. Chem., 132(13), 5249–5255. DOI
- Osterrieth, J. W., Fairen-Jimenez, D. (2021) Metal–organic frameworkcomposites for theragnostics and drug delivery applications. Biotechnol J.,16(2), 2000005. DOI
- Gu, Z.-Y., Yang, C.-X., Chang, N., Yan, X.-P. (2012) Metal–organic frameworks for analytical chemistry: from sample collection tochromatographic separation. Acc. Chem. Res, 45(5), 734–745. DOI
- Gu, Z.-Y., Wang, G., Yan, X.-P. (2010) MOF-5 metal – organic frameworkas sorbent for in-field sampling and preconcentration in combination withthermal desorption GC/MS for determination of atmospheric formaldehyde.Anal Chem., 82(4), 1365–1370. DOI
- Wang, Z., Fu, Y., Kang, Z., Liu, X., Chen, N., Wang, Q., Tu, Y., Wang,L., Song, S., Ling, D. (2017) Organelle-specific triggered release ofimmunostimulatory oligonucleotides from intrinsically coordinated DNA–metal–organic frameworks with soluble exoskeleton. J. Am. Chem. Soc,139(44), 15784–15791. DOI
- Riccò R., Liang W., Li S., Gassensmith J. J., Caruso F., Doonan C.,Falcaro P. (2018) Metal–organic frameworks for cell and virus biology: aperspective. ACS Nano, 12(1), 13–23. DOI
- Li, R., Qu, X., Zhang, Y., Han, H., Li, X. (2016) Lanthanide–organicframeworks constructed from naphthalenedisulfonates: structure, luminescenceand luminescence sensing properties. CrystEngComm, 18, 5890. DOI
- Wu, M. X., Yang, Y. W. (2017) Metal–organic framework (MOF)-baseddrug/cargo delivery and cancer therapy. Adv. Mater., 29(23), 1606134. DOI
- Ren, H., Zhang, L., An, J., Wang, T., Li, L., Si, X., He, L., Wu, X., Wang, C.,Su, Z. (2014) Polyacrylic acid@ zeolitic imidazolate framework-8 nanoparticleswith ultrahigh drug loading capability for pH-sensitive drug release. Chem.Commun., 50(8), 1000–1002. DOI
- Bian, R., Wang, T., Zhang, L., Li, L., Wang, C. (2015) A combination of trimodalcancer imaging and in vivo drug delivery by metal–organic frameworkbased composite nanoparticles. Biomaterial. Sci., 3(9), 1270–1278. DOI
- Zhuang, J., Kuo, C.-H., Chou, L.-Y., Liu, D.-Y., Weerapana, E., Tsung,C.-K. (2014) Optimized Metal–Organic-Framework Nanospheres for DrugDelivery: Evaluation of Small-Molecule Encapsulation. ACS Nano, 8, 3,2812–2819. DOI
- Zhang, H., Chen, W., Gong, K., Chen, J. (2017) Nanoscale zeoliticimidazolate framework-8 as efficient vehicles for enhanced delivery of CpGoligodeoxynucleotides. ACS Appl. Mater. Interfaces, 9(37), 31519–31525. DOI
- Jiang, W., Zhang, H., Wu, J., Zhai, G., Li, Z., Luan, Y., Garg, S. (2018)CuS@MOF-Based Well-Designed Quercetin Delivery System for Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces, 10, 34513. DOI
- Lyu, F., Zhang, Y., Zare, R. N., Ge, J., Liu, Z. (2014) One-Pot Synthesisof Protein-Embedded Metal–Organic Frameworks with Enhanced BiologicalActivities. Nano Letters, 14(10), 5761–5765. DOI
- Abdelhamid, H. N. (2021) Zeolitic Imidazolate Frameworks (ZIF-8) forBiomedical Applications: A Review. Curr. Med. Chem., 28(34), 7023-7075. DOI
- Pan, Y. B., Wang, S., He, X., Tang, W., Wang, J., Shao, A., Zhang, J. (2019)A combination of glioma in vivo imaging and in vivo drug delivery by metalorganicframework based composite nanoparticles. J. Mater. Chem. B, 7(48),7683-7689. DOI
- Zhuang, D., Zhang, H., Genwen, Hu, G., Guo, B. (2022) Recentdevelopment of contrast agents for magnetic resonance and multimodal imagingof glioblastoma. J. Nanobiotechnology, 20(1), 284. DOI
- de Kraker, M. E. A., Stewardson, A. J., Harbarth, S. (2016) Will 10 MillionPeople Die a Year due to Antimicrobial Resistance by 2050? PLoS Med.,13(11), e1002184. DOI
- Anim, A., Mahmoud, L. A. M., Kelly, A. L., Katsikogianni, M. G., Nayak,S. (2023) Biodegradable Polymer Composites of Metal Organic Framework-5(MOF-5) for the Efficient and Sustained Delivery of Cephalexin andMetronidazole. Appl. Sci., 13(19), 10611. DOI
- Baptista, P. V., McCusker, M. P., Carvalho, A., Ferreira, D. A., Mohan, N.M., Martins, M., Fernandes, A. R. (2018) Nano-Strategies to Fight MultidrugResistant Bacteria-“A Battle of the Titans”. Front. Microbiol., 9, 1441. DOI
- Zhang, S., Ye, J., Liu, X., Wang, Y., Li, C., Fang, J., Chang, B., Qi, Y., Li, Y.,Ning, G. (2021) Titanium carbide/zeolite imidazole framework-8/polylactic acidelectrospun membrane for near-infrared regulated photothermal/photodynamictherapy of drug-resistant bacterial infections. J. Colloid. Interf. Sci., 599,390–403. DOI
- Seidi, F., Shamsabadi, A. A., Firouzjaei, M. D., Elliott, M., Saeb, M. R.,Huang, Y., Li, C., Xiao, H., Anasori, B. (2023) MXenes Antibacterial Propertiesand Applications: A Review and Perspective, 19(14), 2206716. DOI
- Zhou, X. M., Shen, Z. Y., Wu, Y. X., Lin, S., Wang, M. D., Xu, T., Wang, L.L., Sadiq, S., Jiao, X. H., Wu, P. (2024) Development of a rapid visual detectiontechnology for BmNPV based on CRISPR/Cas13a system. J. Invertebr. Pathol.,203, 108072. DOI
- Liu, F., Peng, J., Lei, Y.-M., Liu, R.-S., Jin, L., Liang, H., et al. (2022)Electrochemical detection of ctDNA mutation in non-small cell lung cancerbased on CRISPR/Cas12a system. Sens. Actuators B Chem., 362, 131807. DOI
- Yuan, B., Yuan, C., Li, L., Long, M., Chen, Z. (2022) Application of theCRISPR/Cas System in Pathogen Detection: A Review. Molecules, 27(20),6999. DOI
- Li, H., Yang, J., Wu, G., Weng, Z., Song, Y., Zhang, Y., Vanegas, J. A.,Avery, L., Gao, Z., Sun, H., Chen, Y., Dieckhaus, K. D. (2022) Amplification-Free Detection of SARS-CoV-2 and Respiratory Syncytial Virus Using CRISPRCas13a and Graphene Field-Effect Transistors. Angew. Chem. Int. Ed., 61(32),e202203826. DOI
- Zhang, X., Li, Z., Yang, L., Hu, B., Zheng, Q., Man, J., Cao, (2024) J.CRISPR/Cas12a-Derived Photoelectrochemical Aptasensor Based on AuNanoparticle-Attached CdS/UiO-66-NH2 Heterostructures for the Rapid andSensitive Detection of Ochratoxin A. J. Agric. Food. Chem., 72(1), 874-882. DOI
- Du, H., Yin, T., Wang, J., Jie, G. (2023) MultifunctionalPhotoelectrochemical Biosensor Based on ZnIn2S4/ZnS QDs@Au-Ag-Reversed Photocurrent of Cu-Metal-Organic Framework Coupled withCRISPR/Cas-12a-Shearing for Assay of Dual Targets. Anal. Chem., 95(17),7053-7061. DOI
- Kong, L., Zong, C., Chen, X., Xv, H., Lv, M., Li, C. (2024) CRISPR/Cas12atrans-cleavage mediated photoelectrochemical biosensor based on zeoliticimidazolate framework-67 for ATP determination. Mikrochim. Acta, 191(7),403. DOI
- Yan, X., Li, H., Yin, T., Jie, G., Zhou, H. (2022) Photoelectrochemicalbiosensing platform based on in situ generated ultrathin covalent organicframework film and AgInS2 QDs for dual target detection of HIV and CEA.Biosens. Bioelectron., 217, 114694. DOI
- Mousavi, S. M., Hashemi, S. A., Nezhad, F. F., Binazadeh, M.,Dehdashtijahromi, M., Omidifar, N., Ghahramani, Y., Lai, C. W., Chiang, W.-H.,Gholami, A. (2023) Innovative Metal-Organic Frameworks for Targeted OralCancer Therapy: A Review. Materials (Basel), 16(13), 4685. DOI
- Cai, M., Ni, B., Hu, X., Wang, K., Yin, D., Chen, G., Fu, T., Zhu, R., Dong,X., Qu, C., et al. (2022) An investigation of IRMOF-16 as a pH-responsivedrug delivery carrier of curcumin. J. Sci. Adv. Mater. Devices, 7, 100507. DOI
- Tan, G., Zhong, Y., Yang, L., Jiang, Y., Liu, J., Ren, F. (2020) Amultifunctional MOF-based nanohybrid as injectable implant platform for drugsynergistic oral cancer therapy. Chem. Eng. J., 390, 124446. DOI
- Yang, K., Yang, K., Chao, S., Wen, J., Pei, Y., Pei, Z. (2018) Asupramolecular hybrid material constructed from pillar [6] arene-based hostguestcomplexation and ZIF-8 for targeted drug delivery. Chem. Commun., 54,9817-9820. DOI
- Wu, M.-X., Yan, H.-J., Gao, J., Cheng, Y., Yang, J., Wu, J.-R. et al. (2018)Multifunctional Supramolecular Materials Constructed from Polypyrrole@UiO-66 Nanohybrids and Pillararene Nanovalves for Targeted ChemophotothermalTherapy. ACS Appl. Mater. Interfaces, 10, 34655-34663. DOI
- Wu, X., Zhang, Y., Lu, Y., Pang, S., Yang, K., Tian, Z. et al. (2017)Synergistic and targeted drug delivery based on nano-CeO2 capped withgalactose functionalized pillar[5]arenevia host-guest interactions. J. Mater.Chem. B, 5, 3483-3487. DOI
- Wu, M., Gao, J., Wang, F., Yang, J., Song, N., Jin, X. et al. (2018)Multistimuli Responsive Core-Shell Nanoplatform Constructed from Fe3O4@MOF Equipped with Pillar[6]arene Nanovalves. Small, 14, 1704440. DOI
- Yu, G., Yang, J., Fu, X., Wang, Z., Shao, L., Mao, Z. et al. (2018)Supramolecular Hybrid Material Constructed from Graphene Oxide andPillar[6]arene-Based Host-Guest Complex as a Ultrasound and PhotoacousticSignals Nanoamplifier. Mater. Horiz, 5, 429-435. DOI
- Yao, Y., Wang, Y., Huang, F. (2014) Synthesis of various supramolecularhybrid nanostructures based on pillar[6]arene modified gold nanoparticles/nanorods and their application in pH- and NIR-triggered controlled release.Chem. Sci., 5, 4312-4316. DOI. org/10.1039/C4SC01647E
- Tan, X., Zhang, Z., Cao, T., Zeng, W., Huang, T., Zhao, G. (2019)Control Assembly of Pillar[6]arene-Modified Ag Nanoparticles on CovalentOrganic Framework Surface for Enhanced Sensing Performance towardParaquat. ACS Sustain. Chem. Eng. 7(24), 20051–20059. DOI
- Zhang, Y., Li, Q., Liu, C., Shan, X., Chen, X., Dai, W., et al. (2018) Thepromoted effect of a metal–organic frameworks (ZIF-8) on Au/TiO2 for COoxidation at room temperature both in dark and under visible light irradiation.Appl. Catal. B, 224, 283–294. DOI
- Wang, W., Ibarlucea, B. C., Huang, R., Dong, Al., Aiti, M., Huang, S.,Cuniberti, G. (2024) Multi-metallic MOF based composites for environmentalapplications: synergizing metal centers and interactions. Nanoscale Horizons, 9,1432-1474. DOI
- Ahmadijokani, F., Ghaffarkhah, A., Molavi, H., Dutta, S., Yi, Lu, Wuttke,S., Kamkar, M., Rojas, O. J., Arjmand, M. (2024) COF and MOF Hybrids:Advanced Materials for Wastewater Treatment. Adv. Funct. Mater., 34(43),2305527. DOI
- Machado, T. F., Serra, M. E. S., Murtinho, D., Valente, A. J. M.,Naushad, M. (2021) Covalent Organic Frameworks: Synthesis, Propertiesand Applications—An Overview. Polymers, 13(6), 970. DOI