THE EFFECT OF THE MATRICELLULAR PROTEIN TENASCIN-C ON THE FUNCTIONAL ACTIVITY OF FIBROBLASTS IN AN EXPERIMENTAL IN VITRO INJURY MODEL
Kuban State Medical University, 4 Mitrofana Sedina str., 350063, Krasnodar, Russia; e-mail: cnil@ksma.ru
Keywords:tenascin-C; fibroblasts; wound healing; matricellular proteins, collagenogenesis; alarmin
DOI:10.18097/BMCRM00286
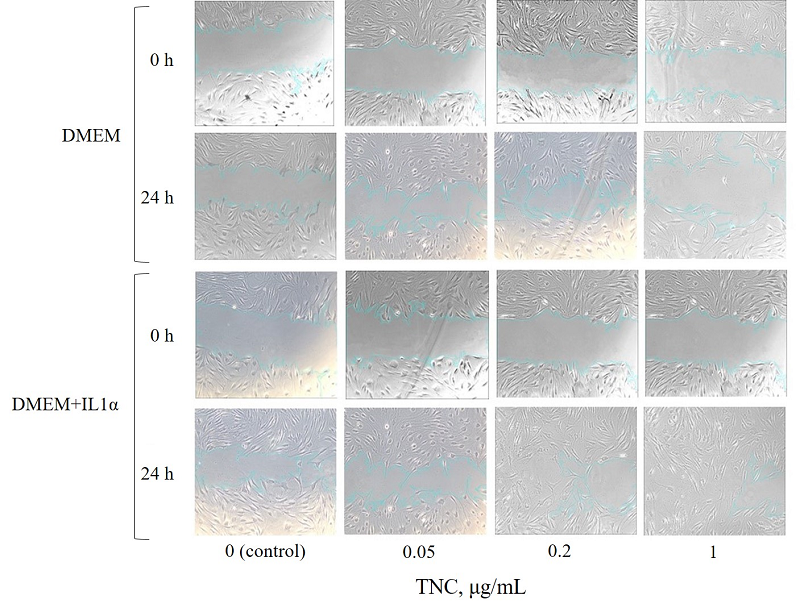
Wound healing is a complex, multistep process involving sequential phases of inflammation, proliferation, and remodeling. Tenascin-C (TNC) is a matricellular protein actively involved in tissue regeneration. It is upregulated in response to tissue injury and plays an important role in the regulation of cell adhesion, migration, proliferation, and extracellular matrix protein synthesis. At the same time, during the early stages of wound healing, interleukin-1α (IL1α) exerts a significant effect as an alarmin that initiates inflammatory activation of fibroblasts. The objective of this study was to determine the optimal concentration of TNC stimulating both the migratory and synthetic activity of human dermal fibroblasts in vitro, including under conditions of preliminary inflammatory activation with IL1α. To this end, a comparative analysis of cell migration and proliferation was conducted, along with measurement of type I collagen synthesis using DF-1 human fibroblast cultures pre-incubated with IL1α (50 ng/mL) for 24 h, followed by the addition of recombinant TNC at concentrations of 0.05 μg/ml, 0.2 μg/ml, and 1 μg/mL. TNC exhibited a dose-dependent effect on fibroblasts: at a concentration of 0.2 µg/ml it stimulates cell migration and proliferation, accompanied by a statistically significant increase in type I collagen synthesis compared with the control. However, this level was lower than that observed at 1 µg/mL TNC, where a marked increase in collagen production was detected. Under conditions of IL1α pre-stimulation, the effects of TNC were amplified, particularly at concentrations of 0.2 μg/ml and 1 μg/ml, indicating the potential of TNC as a regulator of cellular activity within an inflammatory microenvironment. Higher concentrations did not further increase the effect. These findings may relevant in the context of to the development of biomaterials and therapeutic agents aimed at accelerating cutaneous wound healing by modulating cellular activity, which is especially relevant for the treatment of chronic or non-healing wounds.

|
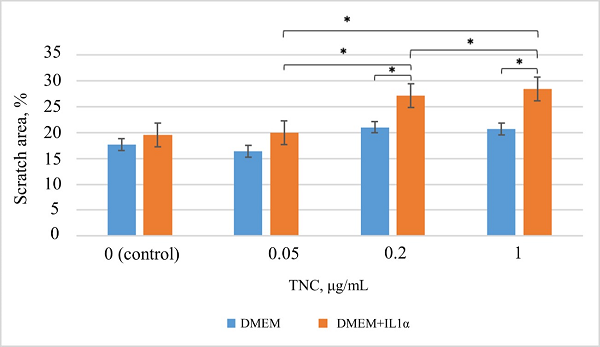
Figure 2.
Results of the analysis of migratory activity under TNC exposure according to the scratch assay. * – the differences are statistically significant, p < 0.05.
|

|
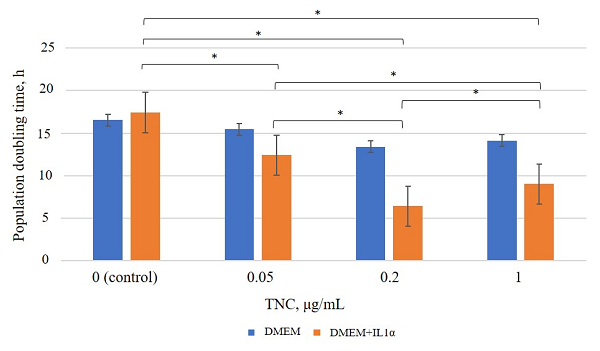
Figure 3.
Population doubling time under TNC exposure. * – the differences are statistically significant, p < 0.05.
|

|
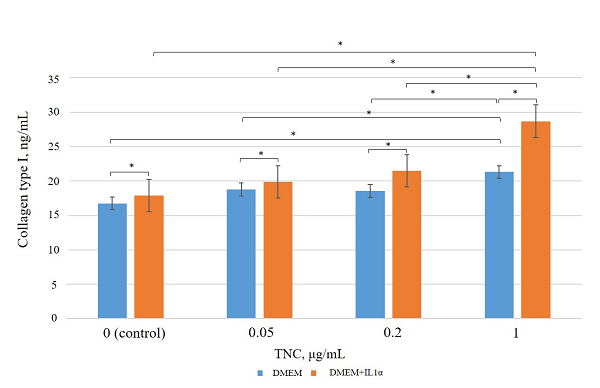
Figure 4.
Type I collagen levels in conditioned medium after incubation of dermal fibroblasts with TNC. * – the differences are statistically significant, p < 0.05.
|
FUNDING
This study was supported by the Russian Science Foundation, https://rscf.ru/en/project/24-25-20083/ grant No. 24-25-20083, and co-funded by the Kuban Science Foundation under project No. 24-25-20083.
REFERENCES
- Chiquet-Ehrismann, R., Tucker, R.P. (2011) Tenascins and the importanceof adhesion modulation. Cold Spring Harbor Perspectives in Biology, 3(5),a004960. DOI
- Tracy, L.E., Minasian, R.A., Caterson, E.J. (2016) Extracellular matrix anddermal fibroblast function in the healing wound. Adv Wound Care, 5(3), 119-136. DOI
- Boraldi, F., Lofaro, F.D., Bonacorsi, S., Mazzilli, A., Garcia-Fernandez, M.,Quaglino, D. (2024) The role of fibroblasts in skin homeostasis and repair.Biomedicines, 12(7). 1586. DOI
- Mathew-Steiner, S.S., Roy, S., Sen, C.K. (2021) Collagen in wound healing.Bioengineering, 8(5), 63. DOI
- Wang, Y., Wang, G., Liu H. (2022) Tenascin-C: a key regulator inangiogenesis during wound healing. Biomolecules, 12(11), 1689. DOI
- Rock, K.L., Latz, E., Ontiveros, F., Kono, H. (2010) The sterile inflammatoryresponse. Annual Review of Immunology, 28, 321-342. DOI
- Midwood, K.S., Orend, G. (2009) The role of tenascin-C in tissue injury andtumorigenesis. Journal of Cell Communication and Signaling, 3(3-4), 287-310. DOI
- Glotzbach K., Faissner A. (2024) Substrate-bound and soluble domains oftenascin-C regulate differentiation, proliferation and migration of neural stemand progenitor cells. Frontiers in Cellular Neuroscience, 18, 1357499. DOI
- Cavalli, G., Colafrancesco, S., Emmi, G., Imazio, M., Lopalco, G., Maggio,M.C., Sota J., Dinarello, C.A (2021) Interleukin 1α: A comprehensive reviewon the role of IL-1α in the pathogenesis and treatment of autoimmune andinflammatory diseases. Autoimmunity Reviews, 20(3), 102763. DOI
- Kim, B., Lee, Y., Kim, E., Kwak, A., Ryoo, S., Bae, S. H., Azam, T., Kim, S.,Kim, S., Dinarello, C.A. (2013) The interleukin-1α precursor is biologicallyactive and is likely a key alarmin in the IL-1 family of cytokines. Frontiers inimmunology, 4, 391. DOI
- Bahar, E., Yoon, H. (2021) Modeling and predicting the cell migrationproperties from scratch wound healing assay on cisplatin-resistant ovariancancer cell lines using artificial neural network. Healthcare, 9(7), 911. DOI
- Sharma, R.E.K.H.A., Sharma, H., Ahlawat, S., Tantia, M.S. (2018) Anefficient method of generating skin fibroblast cells for cell banking. IndianJournal of Animal Sciences, 88(8), 905-909. DOI
- Choi, Y.E., Song, M.J., Hara, M., Imanaka-Yoshida, K., Lee, D.H., Chung,J.H., Lee, S.T. (2020) Effects of tenascin C on the integrity of extracellularmatrix and skin aging. International Journal of Molecular Sciences, 21(22),8693. DOI
- Radwanska, A., Grall, D., Schaub, S., Divonne, S.B.D.L.F., Ciais, D.,Rekima, S., Rupp, T., Sudaka, A., Orend, G., Van Obberghen-Schilling, E.(2017) Counterbalancing anti‑adhesive effects of tenascin-C through fibronectinexpression in endothelial cells. Scientific Reports, 7(1), 12762. DOI
- Katoh, D., Kozuka, Y., Noro, A., Ogawa, T., Imanaka-Yoshida, K.,Yoshida, T. (2020) Tenascin‑C induces phenotypic changes in fibroblasts tomyofibroblasts with high contractility through the integrin αvβ1/transforminggrowth factor-β/SMAD signaling axis in human breast cancer. The AmericanJournal of Pathology, 190(10), 2123-2135. DOI
- Dinarello, C.A. (2018) Overview of the IL-1 family in innate inflammationand acquired immunity. Immunological Reviews, 281(1), 8-27. DOI
- Giménez, A., Duch, P., Puig, M., Gabasa, M., Xaubet, A., Alcaraz, J.(2017) Dysregulated collagen homeostasis by matrix stiffening and TGF-β1in fibroblasts from idiopathic pulmonary fibrosis patients: Role of FAK/Akt .International journal of molecular sciences. 18(11), 2431. DOI
- Talbott, H.E. Mascharak, S., Griffin, M., Wan, D.C., Longaker, M.T. Woundhealing, fibroblast heterogeneity, and fibrosis (2022). Cell stem cell, 29(8),1161-1180. DOI
- Wynn, T.A., Ramalingam, T.R. (2012) Mechanisms of fibrosis: Therapeutictranslation for fibrotic disease. Nature Medicine, 18(7), 1028-1040. DOI