The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Evolution of methods for assessing the motor function of laboratory rodents - neurodegenerative diseases models
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: chicheva.mariya@gmail.com
Key words: neurodegeneration; gait; animal models; motor function
DOI: 10.18097/BMCRM00030
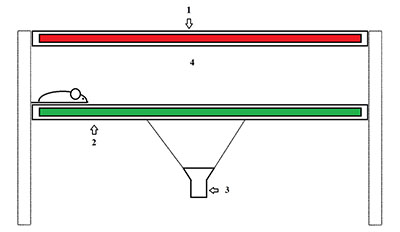
This review contains information about different laboratorian rodent’s gait analysis systems. These methods are useful for the assessment of motor function in neurodegenerative models. The following aspects have been considered: ink traces technique, treadmills equipment, and modern gait analysis systems like TreadScan and CatWalk, which allows estimating a set of animals gait parameters. For each technique a detailed description and examples of its use for estimating gait parameters in neurodegenerative diseases are given.
ACKNOWLEDGEMENTS
The study was support by RFBR (No. 16-04-01089); transgenic animals used in the study were provided by Institute of Physiologically Active Compounds of the Russian Academy of Sciences «Transgen» collection, developed within project No. 0090-2017-0016; the study was conducted using equipment of Center for collective use of IPAC RAS in the framework of the State Scientific Assignment to IPAC RAS (No. 0090-2017-0019) and the program of the Russian Academy of Sciences the Theme №48.8. Search and research of mechanisms of action of neuroprotectors and stimulators of cognitive functions.
REFERENCES
- Arendt, T., Mosch, B., Morawski, M. (2009). Neuronal aneuploidy in health and disease: a cytomic approach to understand the molecular individuality of neurons. Int J Mol Sci, 10(4), 1609-1627. DOI
- Kline, R. A., Kaifer, K. A., Osman, E. Y., Carella, F., Tiberi, A., Ross, J., Pennetta, G., Lorson, C. L., Murray, L. M. (2017). Comparison of independent screens on differentially vulnerable motor neurons reveals alpha-synuclein as a common modifier in motor neuron diseases. PLoS Genet, 13(3), e1006680. DOI
- Gonzalez-Gomez, I., Mononen, I., Heisterkamp, N., Groffen, J., Kaartinen, V. (1998). Progressive neurodegeneration in aspartylglycosaminuria mice. Am J Pathol, 153(4), 1293-1300. DOI
- Messer, A., Strominger, N. L., Mazurkiewicz, J. E. (1987). Histopathology of the late-onset motor neuron degeneration (Mnd) mutant in the mouse. J Neurogenet, 4(4), 201-213.
- Yin, Z., Valkenburg, F., Hornix, B. E., Mantingh-Otter, I., Zhou, X., Mari, M., Reggiori, F., Van Dam, D., Eggen, B. J. L., De Deyn, P. P., Boddeke, E. (2017). Progressive Motor Deficit is Mediated by the Denervation of Neuromuscular Junctions and Axonal Degeneration in Transgenic Mice Expressing Mutant (P301S) Tau Protein. J Alzheimers Dis, 60(s1), S41-S57. DOI
- Shelkovnikova, T. A., Peters, O. M., Deykin, A. V., Connor-Robson, N., Robinson, H., Ustyugov, A. A., Bachurin, S. O., Ermolkevich, T. G., Goldman, I. L., Sadchikova, E. R., Kovrazhkina, E. A., Skvortsova, V. I., Ling, S. C., Da Cruz, S., Parone, P. A., Buchman, V. L., Ninkina, N. N. (2013). Fused in sarcoma (FUS) protein lacking nuclear localization signal (NLS) and major RNA binding motifs triggers proteinopathy and severe motor phenotype in transgenic mice. J Biol Chem, 288(35), 25266-25274. DOI
- D'Hooge, R., Hartmann, D., Manil, J., Colin, F., Gieselmann, V., De Deyn, P. P. (1999). Neuromotor alterations and cerebellar deficits in aged arylsulfatase A-deficient transgenic mice. Neurosci Lett, 273(2), 93-96.
- Fernagut, P. O., Diguet, E., Labattu, B., Tison, F. (2002). A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods, 113(2), 123-130.
- de Medinaceli, L., Freed, W. J., Wyatt, R. J. (1982). An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental neurology, 77(3), 634-643.
- Wilson, J. M., Petrik, M. S., Moghadasian, M. H., Shaw, C. A. (2005). Examining the interaction of apo E and neurotoxicity on a murine model of ALS-PDC. Can J Physiol Pharmacol, 83(2), 131-141. DOI
- Takayanagi, N., Beppu, H., Mizutani, K., Tomita, Y., Nagao, S., Suzuki, S., Orand, A., Takahashi, H., Sonoda, S. (2013). Pelvic axis-based gait analysis for ataxic mice. J Neurosci Methods, 219(1), 162-168. DOI
- Guillot, T. S., Asress, S. A., Richardson, J. R., Glass, J. D., Miller, G. W. (2008). Treadmill gait analysis does not detect motor deficits in animal models of Parkinson's disease or amyotrophic lateral sclerosis. J Mot Behav, 40(6), 568-577. DOI
- Amende, I., Kale, A., McCue, S., Glazier, S., Morgan, J. P., Hampton, T. G. (2005). Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J Neuroeng Rehabil, 2, 20. DOI
- Vinsant, S., Mansfield, C., Jimenez-Moreno, R., Del Gaizo Moore, V., Yoshikawa, M., Hampton, T. G., Prevette, D., Caress, J., Oppenheim, R. W., Milligan, C. (2013). Characterization of early pathogenesis in the SOD1(G93A) mouse model of ALS: part II, results and discussion. Brain Behav, 3(4), 431-457. DOI
- Beare, J. E., Morehouse, J. R., DeVries, W. H., Enzmann, G. U., Burke, D. A., Magnuson, D. S., Whittemore, S. R. (2009). Gait analysis in normal and spinal contused mice using the TreadScan system. J Neurotrauma, 26(11), 2045-2056. DOI
- Vrinten, D. H. Hamers, F. F. (2003). 'CatWalk' automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain, 102(1-2), 203-209.
- Jakeman, L. B., Chen, Y., Lucin, K. M., McTigue, D. M. (2006). Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur J Neurosci, 23(8), 1997-2011. DOI
- Van Meeteren, N. L., Eggers, R., Lankhorst, A. J., Gispen, W. H., Hamers, F. P. (2003). Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma, 20(10), 1029-1037. DOI
- Abu-Ghefreh, A. A. Masocha, W. (2010). Enhancement of antinociception by coadministration of minocycline and a non-steroidal anti-inflammatory drug indomethacin in naive mice and murine models of LPS-induced thermal hyperalgesia and monoarthritis. BMC Musculoskelet Disord, 11, 276. DOI
- Ferreira-Gomes, J., Adaes, S., Castro-Lopes, J. M. (2008). Assessment of movement-evoked pain in osteoarthritis by the knee-bend and CatWalk tests: a clinically relevant study. J Pain, 9(10), 945-954. DOI
- Vandeputte, C., Taymans, J. M., Casteels, C., Coun, F., Ni, Y., Van Laere, K., Baekelandt, V. (2010). Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci, 11, 92. DOI
- Casteels, C., Vandeputte, C., Rangarajan, J. R., Dresselaers, T., Riess, O., Bormans, G., Maes, F., Himmelreich, U., Nguyen, H., Van Laere, K. (2011). Metabolic and type 1 cannabinoid receptor imaging of a transgenic rat model in the early phase of Huntington disease. Exp Neurol, 229(2), 440-449. DOI
- Chiang, M. C., Chen, C. M., Lee, M. R., Chen, H. W., Chen, H. M., Wu, Y. S., Hung, C. H., Kang, J. J., Chang, C. P., Chang, C., Wu, Y. R., Tsai, Y. S., Chern, Y. (2010). Modulation of energy deficiency in Huntington's disease via activation of the peroxisome proliferator-activated receptor gamma. Hum Mol Genet, 19(20), 4043-4058. DOI
- Barber, S. C. Shaw, P. J. (2010). Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med, 48(5), 629-641. DOI
- Gerber, Y. N., Sabourin, J. C., Rabano, M., Vivanco, M., Perrin, F. E. (2012). Early functional deficit and microglial disturbances in a mouse model of amyotrophic lateral sclerosis. PLoS One, 7(4), e36000. DOI
- Mead, R. J., Bennett, E. J., Kennerley, A. J., Sharp, P., Sunyach, C., Kasher, P., Berwick, J., Pettmann, B., Battaglia, G., Azzouz, M., Grierson, A., Shaw, P. J. (2011). Optimised and rapid pre-clinical screening in the SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis (ALS). PLoS One, 6(8), e23244. DOI
- Nanou, A., Higginbottom, A., Valori, C. F., Wyles, M., Ning, K., Shaw, P., Azzouz, M. (2013). Viral delivery of antioxidant genes as a therapeutic strategy in experimental models of amyotrophic lateral sclerosis. Mol Ther, 21(8), 1486-1496. DOI
- Eleftheriadou, I., Manolaras, I., Irvine, E. E., Dieringer, M., Trabalza, A., Mazarakis, N. D. (2016). alphaCAR IGF-1 vector targeting of motor neurons ameliorates disease progression in ALS mice. Ann Clin Transl Neurol, 3(10), 752-768. DOI
- Lee, J. K., Shin, J. H., Hwang, S. G., Gwag, B. J., McKee, A. C., Lee, J., Kowall, N. W., Ryu, H., Lim, D. S., Choi, E. J. (2013). MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proc Natl Acad Sci U S A, 110(29), 12066-12071. DOI
- Mead, R. J., Higginbottom, A., Allen, S. P., Kirby, J., Bennett, E., Barber, S. C., Heath, P. R., Coluccia, A., Patel, N., Gardner, I., Brancale, A., Grierson, A. J., Shaw, P. J. (2013). S[+] Apomorphine is a CNS penetrating activator of the Nrf2-ARE pathway with activity in mouse and patient fibroblast models of amyotrophic lateral sclerosis. Free Radic Biol Med, 61, 438-452. DOI
- Audouard, E., Schakman, O., Rene, F., Huettl, R. E., Huber, A. B., Loeffler, J. P., Gailly, P., Clotman, F. (2012). The Onecut transcription factor HNF-6 regulates in motor neurons the formation of the neuromuscular junctions. PLoS One, 7(12), e50509. DOI
- Vergouts, M., Marinangeli, C., Ingelbrecht, C., Genard, G., Schakman, O., Sternotte, A., Calas, A. G., Hermans, E. (2015). Early ALS-type gait abnormalities in AMP-dependent protein kinase-deficient mice suggest a role for this metabolic sensor in early stages of the disease. Metab Brain Dis, 30(6), 1369-1377. DOI
- Scekic-Zahirovic, J., Oussini, H. E., Mersmann, S., Drenner, K., Wagner, M., Sun, Y., Allmeroth, K., Dieterle, S., Sinniger, J., Dirrig-Grosch, S., Rene, F., Dormann, D., Haass, C., Ludolph, A. C., Lagier-Tourenne, C., Storkebaum, E., Dupuis, L. (2017). Motor neuron intrinsic and extrinsic mechanisms contribute to the pathogenesis of FUS-associated amyotrophic lateral sclerosis. Acta Neuropathol, 133(6), 887-906. DOI
- Scekic-Zahirovic, J., Sendscheid, O., El Oussini, H., Jambeau, M., Sun, Y., Mersmann, S., Wagner, M., Dieterle, S., Sinniger, J., Dirrig-Grosch, S., Drenner, K., Birling, M. C., Qiu, J., Zhou, Y., Li, H., Fu, X. D., Rouaux, C., Shelkovnikova, T., Witting, A., Ludolph, A. C., Kiefer, F., Storkebaum, E., Lagier-Tourenne, C., Dupuis, L. (2016). Toxic gain of function from mutant FUS protein is crucial to trigger cell autonomous motor neuron loss. EMBO J, 35(10), 1077-1097. DOI
- Chedly, J., Soares, S., Montembault, A., Von Boxberg, Y., Veron-Ravaille, M., Mouffle, C., Benassy, M.-N., Taxi, J., David, L., Nothias, F. (2017). Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials, 138, 91-107.