Electrochemical Methods for Studies of Biological Molecules
1Institute of Biomedical Chemistry, 8119121 Pogodinskaya Str., 10 bldg. 8, Moscow, Russia,*e-mail: viktoria.shumyantseva@ibmc.msk.ru 2Biochemistry Department of Medico-Biological faculty Pirogov Russian National Research Medical University, Moscow, Russia 3Lomonosov Moscow State University, Chemical Department, Moscow, Russia
Keywords: electoanalysis; bioelectrochemistry; hemeproteins; DNA; drugs; medicinal preparations; electrooxidation; electrocatalysis
DOI: 10.18097/BMCRM00032
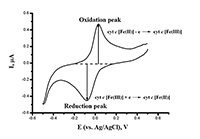
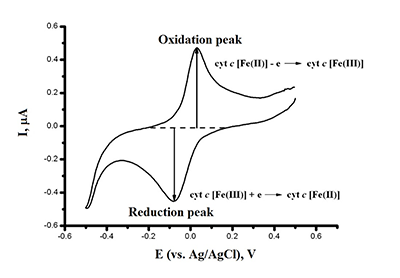
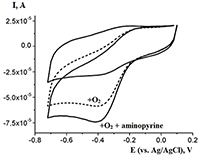
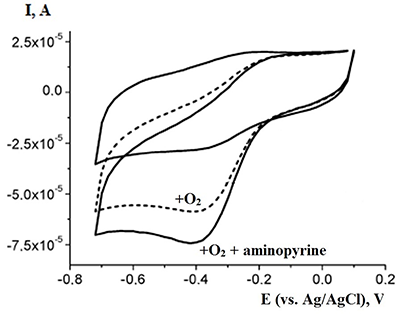
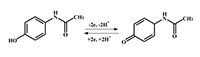
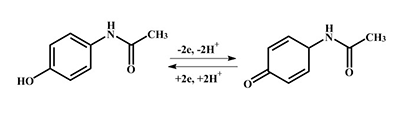
This paper focuses on experimental data of electroanalysis of enzymes, proteins, peptides, DNA, and medicinal preparations, obtained by authors. Methods for enzyme electrodes preparation, methods for kinetic parameters calculations based on analysis of electrochemical data. Results are described as algorithm for efficient electrochemical reaction of biomolecules.
|
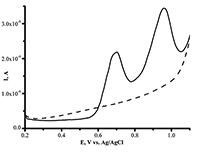
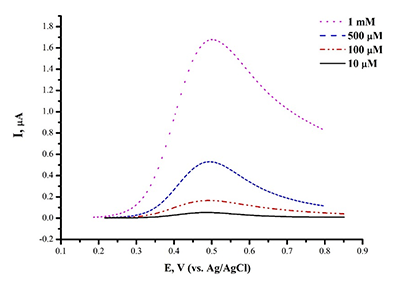
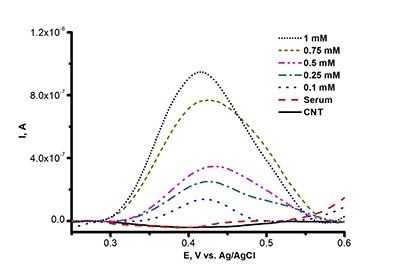
Figure 3a.
Differential pulse voltammetry (DPV), the oxidation curve of SPE/CNT with varied L-tyrosine concentration (0.1 mM ÷ 1 mM) in human serum
|
|
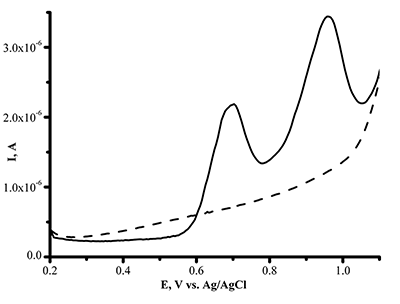
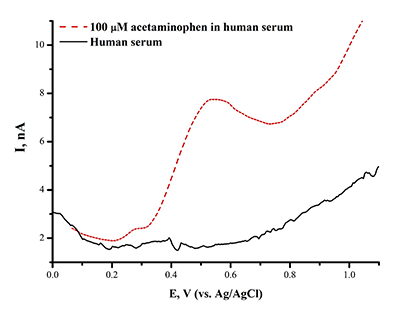
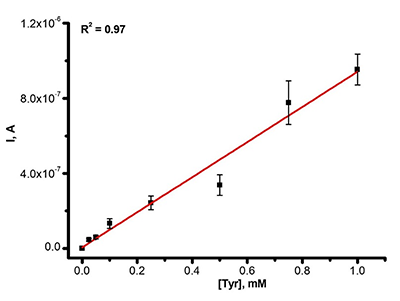
Figure 3b.
The dependence of DPV oxidation current intensity of SPE/CNT on the L-tyrosine concentration in human serum
|
|
CLOSE

|
Table 1.
Comparative characteristics of electrochemical systems
|
ACKNOWLEDGEMENTS
This study was supported by the Russian Foundation for Basic Research (project no. 18-04-00374) and within the framework of the Fundamental Research Program of State Academies of Sciences for 2013-2020.
REFERENCES
- Bard, A.E. (2001) Electrochemical methods. Fundamental and Applications, A.E. Bard, L.R. Faulkner. NY: Eds John Wiley & Sons, Second edition
- Scholz, F. (2002) Electroanalytical methods. Guide to Experiments and Applications. Springer-Verlag Berlin Heidelberg
- Wang, J. (2006) Analytical Electrochemistry, Third edition, p. 32, Wiley-VCH
- Compton, R., & Banks, C. (2011) Understanding voltammetry. (2nd edition), by Imperial College Press
- Laviron, E. (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. Journal of Electroanalytical Chemistry, 101, 19-28. DOI
- Shumkov, A.A., Suprun, E.V., Shatinina, S.Z., Lisitsa, A.V., Shumyantseva, V.V., & Archakov, A.I. (2013) Gold and Silver Nanoparticles for Electrochemical Detection of Cardiac Troponin I based on Striping Voltammetry. BioNanoScience, 3, 216-222. DOI
- Hrycay, E.G., & Bandiera, S.M. (2015) Monooxygenase, peroxygenase and peroxidase properties and reaction mechanism of cytochrome P450 enzymes. pp. 1-61. In EG Hrycay and SM Bandiera (eds.), Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450. Advances in Experimental Medicine and Biology 851. Springer International Publishing SwitzerlandDOI
- Shumyantseva, V.V., Ivanov, Yu.D., Bistolas, N., Scheller, F.W., Archakov, A.I., & Wollenberger, U. (2004) Direct Electron Transfer of Cytochrome P450 2B4 at Electrodes Modified with Nonionic Detergent and Colloidal Clay Nanoparticles. Analytical Chemistry, 76, 6046-6052. DOI
- Shumyantseva, V.V., Bulko, T., Shich, E., Makhova, A., Kuzikov, A., & Archakov, A.A. (2015) Cytochrome P450 Enzymes and Electrochemistry: Crosstalk with Electrodes as Redox Partners and Electron Sources. pp. 229-246. In EG Hrycay and SM Bandiera (eds.), Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450. Advances in Experimental Medicine and Biology 851. Springer International Publishing Switzerland. DOI
- Bartlett, P.N. (Ed.), (2008) Bioelectrochemistry: Fundametals, Experimental Techniques and Applications, Ch. 2, John Wiley & Sons, Ltd., p. 268
- Hagen, K.D., Gillan, J.M., Im, S.-C., Landefeld, S., Mead, G., Hiley, M., et al. (2013). Electrochemistry of mammalian cytochrome P450 2B4 indicates tunable thermodynamic parameters in surfactant films. Journal of Inorganic Biochemistry, 129, 30-34. DOI
- Shumyantseva, V.V., Suprun, E.V., Bulko, T.V., & Archakov, A.I. (2014). Electrochemical methods for detection of post-translational modifications of proteins. Biosensors and Bioelectronics, 61, 131-139. DOI
- Brabec, V, & Mornstein, V. (1980). Biochimica et Biophysica Acta, 625, 43-50. DOI
- Reynaud, J.A., Malfoy, B., & Bere, A. (1980). The electrochemical oxidation of three proteins: RNAase A, bovine serum albumin and concanavalin A at solid electrodes. Journal of Electroanalytical and Chemical Interfacial Electrochemistry 116, 595-606. DOI
- Suprun, E.V., Zharkova, M.S., Morozevich, G.E., Veselovsky, A.V., Shumyantseva, V.V. & Archakov, A.I. (2013). Analysis of Redox Activity of Proteins on the Carbon Screen Printed Electrodes. Electronanalysis, 25(9), 2109-2116. DOI
- Shumyantseva, V., Bulko, T., Kuzikov, A., Masamrekh, R., & Archakov, A. (2018). Analysis of L-tyrosine based on electrocatalytic oxidative reactions via screen-printed electrodes modified with multi-walled carbon nanotubes and nanosized titanium oxide (TiO2). Amino Acids, 50, 823-829. DOI
- Radko, S.P., Khmeleva, S.A., Suprun, E.V., Kozin, S.A., Bodoev, N.V., Makarov A.A. et al. (2015). Physico-chemical methods for studying amyloid-β aggregation. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 9(3), 258-274. DOI
- Abo-Hamadb, A., AlSaadi, M.A., Hayyan, M., Juneidi, I., & Hashim, M.A. (2016). Ionic Liquid-Carbon Nanomaterial Hybrids for Electrochemical Sensor Applications: a Review. Electrochimica Acta 193 (2016) 321–343. DOI
- Ren, S., Wang, H., Zhang, H., Yu, L., Li, M., & Li, M. (2015). Direct electrocatalytic and simultaneous determination of purine and pyrimidine DNA bases using novel mesoporous carbon fibers as electrocatalyst. Journal of Electroanalytical Chemistry, 750, 65–73. DOI
- Rahi, A., Karimian, K., & Heli, H. (2016). Nanostructured materials in electroanalysis of pharmaceuticals. Analytical Biochemistry, 497, 39-47. DOI
- Cernat, A., Tertis¸ M., & Sandulescu, R. (2015). Electrochemical sensors based on carbon nanomaterials for acetaminophen detection: A review. Analytica Chimica Acta, 886, 16-28. DOI