The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
SiO2-Based Aerogels Modified by Covalently Bonded Aromatic Acids as Potential Drug Delivery Systems
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: lermontov52@yandex.ru
2Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, 31 Leninsky av., Moscow, 119991 Russia
Key words: aerogels; supercritical drying; surface modification
DOI: 10.18097/BMCRM00037
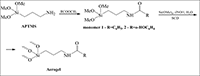
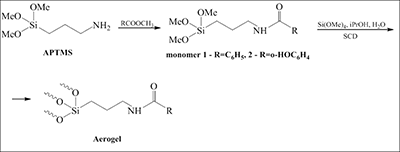
Hybrid aerogels (AGs) containing NH2-group were acylated by benzoic and salicylic acids. The acylated AGs had the specific area value of 170-220 m2/g and were not deacylated in H2O-iPrOH mixture at 37°С during 24 h. In 0.5% HCl at 37°С hydrolysis takes place releasing free acids and giving the possibility to use aminoaerogels as drug delivery system.
ACKNOWLEDGEMENTS
The research was carried out with financial support from the Russian Science Foundation (project no. 14-13-01150, synthesis and studying of the aerogels’ characteristics), hydrolytic resistance tests was accomplish within the state assignment of 2018 (theme No. 0090-2014-0005).
REFERENCES
- Husing, N., & Schubert, U. (2005). Aerogels. In Ullmann’s Encyclopedia of Industrial Chemistry (pp. 748–769). Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. DOI
- Wittwer, V. (1992). Development of aerogel windows. Journal of Non-Crystalline Solids, 145(C), 233–236. DOI
- Alexa, L. C., Huber, G. M., Lolos, G. J., Farzanpay, F., Garibaldi, F., Jodice, M., Leone, A., Perrino, R., Papandreou, Z., Humphrey, D. L., Ulmer, P., DeLeo, R. (1995). Empirical tests and model of a silica aerogel Cherenkov detector for CEBAF. Nuclear Inst. and Methods in Physics Research, A, 365(2–3), 299–307. DOI
- Reynolds, J. G., Coronado, P. R., & Hrubesh, L. W. (2001). Hydrophobic aerogels for oil-spill clean up - Synthesis and characterization. Journal of Non-Crystalline Solids, 292(1–3), 127–137. DOI
- Teichner, S. J., Nicolaon, G. A., Vicarini, M. A., & Gardes, G. E. E. (1976). Inorganic oxide aerogels. Advances in Colloid and Interface Science, 5(3), 245–273. DOI
- I. Smirnova, S. Suttiruengwong, M. Seiler, W. Arlt, Dissolution Rate Enhancement by Adsorption of Poorly Soluble Drugs on Hydrophilic Silica Aerogels, Pharm. Dev. Technol. 9 (2005) 443–452. DOI