The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Protective Action of the Extract of Blueberry Leaves Against Glutamate Excitotoxicity on Neurons of the Rat Cerebral Cortex
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: e-mail: klochkov@ipac.ac.ru
Key words: extract; blueberry; cultured neurons; glutamate; MK-801; excitotoxicity
DOI: 10.18097/BMCRM00045
The aqueous extract of blueberry leaves inhibits the glutamate-induced Ca2 + influx into the synaptosomes of rat brain neurons and the IC50 value of this process is close to the IC50 for MK-801, a well-known noncompetitive antagonist of glutamate NMDA subtype receptors. The aqueous extract of blueberry leaves protected the cultured neurons of the rat cerebral cortex from the neurotoxic effect of glutamate, and the inhibition intensity depended on the incubation time with the extract.
|
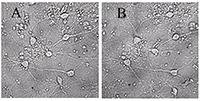
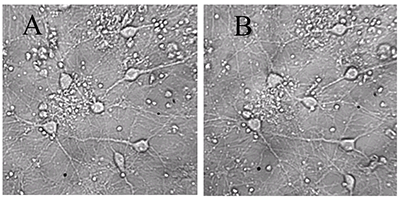
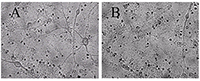
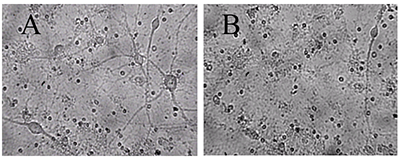
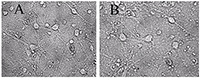
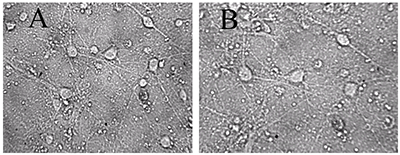
Figure 1.
A - before, B -30 min in a medium with 100 μM glutamate, then in a culture medium for 18 hours before shooting. All the neurons died, except one. (Bright field method).
|
|
CLOSE

|
Table 1.
Effect of MK-801 and blueberry extract on the capture of 45Ca2+ in the synaptosomes of the cerebral cortex when stimulated with glutamate.
|
|
CLOSE

|
Table 2.
Effect of blueberry leaves (Vaccinium myrtillus L.) extract on the survival of cultured neurons under the action of glutamate. The number of live neurons after incubation with the test substances after 18 h (% of control).
|
ACKNOWLEDGEMENTS
The equipment of the Center for Collective Use of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences was used. The work was carried out within the framework of the State Proposal 0090-2017-0018.
REFERENCES
- C. Spagnuolo, M. Napolitano, I. Tedesco, S. Moccia, A. Milito, G.L. Russo. (2016). Neuroprotective Role of Natural Polyphenols. Curr Top Med Chem.;16(17):1943-50. DOI
- S. Zhou, X. Chen, X. Gu, F. Ding. (2009). Achyranthes bidentata Blume extract protects cultured hippocampal neurons against glutamate-induced neurotoxicity. J Ethnopharmacol., 122(3):547-54. DOI
- Y. Shimada, H. Goto, T. Kogure, K. Kohta, T. Shintani, T. Itoh, K. Terasawa. (2000). Extract prepared from the bark of Cinnamomum cassia Blume prevents glutamate-induced neuronal death in cultured cerebellar granule cells. Phytother Res.; 14(6):466-8. DOI
- J. Cho, J.Y. Kong, D.Y. Jeong, K.D. Lee, D.U. Lee, B.S. Kang. (2001). NMDA recepter-mediated neuroprotection by essential oils from the rhizomes of Acorus gramineus. Life Sci.; 68(13):1567-73. DOI
- S.M. Rothman, J.W. Olney. (1986). Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. (2):105-11. DOI
- R. Wang, P.H. Reddy. Role of Glutamate and NMDA Receptors in Alzheimer's Disease. (2017). J Alzheimers Dis.;57(4):1041-1048. DOI
- Y. Qian, X. Tang, T. Guan, Y. Li, H. Sun. (2016). Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model. Molecules. 21(8). pii: E1093. DOI
- Chemical analysis of medicinal plants, ed. N.I. Grinkevich, L.N. Safronich. M., High School, 1983, str.91-92
- Grigoriev, V. V., Petrova, L. N., Gabrelian, A. V., Zamoyski, V. L., Serkova, T. P., & Bachurin, S. O. (2012). Effect of somatostatin on presynaptic and postsynaptic glutamate receptors and postsynaptic GABA receptors in the neurons of rat brain. Bulletin of experimental biology and medicine, 154(1), 10-12. DOI
- B.H. Havsteen. (2002). The biochemistry and medical significance of the flavonoids. Pharmacology & Therapeutics, 96(2-3), 67-202. DOI
- P. Mauri, P. Pietta. (2000). Electrospray characterization of selected medicinal plant extracts. J. of Pharmaceutical and Biomedical Analysis, 23, 61–68. DOI
- R. Tremblay, B. Chakravarthy, K. Hewitt, J. Tauskela, P. Morley, T. Atkinson, J.P. Durkin. (2000). Transient NMDA receptor inactivation provides long-term protection to cultured cortical neurons from a variety of death signals. J Neurosci.; 20(19):7183-92. DOI
- V.A. Chistyakov (2008). Nonspecific Mechanisms for Protection against Destructive Action of Reactive Oxygen Species. Uspekhi sovremennoj biologii. 128(3), 300-306.
- K. Ishige, D. Schubert, Y. Sagara. (2001). Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med.; 30(4):433-46. DOI