The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Development of 3D Cell Culture on Ultra-High Molecular Weight Polyethylene (UHMWPE) as the Basis of Cellular Matrix
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia,*e-mail: alexey@ipac.ac.ru 2National University of Science and Technology, 4 Leninskiy prospekt, Moscow, 119049 Russia
Key words: 3D cell culture modeling; polymers; ultra-high-molecular-weight polyethylene
DOI: 10.18097/BMCRM00048
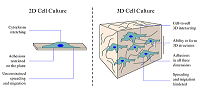
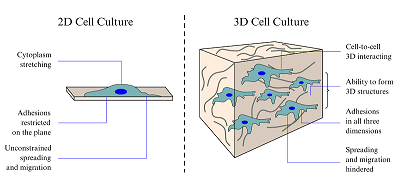
The study is devoted to the development of an artificial material based on the ultrahigh-molecular weight polyethylene (UHMWPE) with a porous or cellular 3D structure as a cellular matrix – a framework for growing cell cultures. The development of such matrix provides support for neuronal cell culture under conditions that mimick those that exist in the living body. Typically, in vitro cellular studies are conducted in a 2D format, which limits intercellular interactions, morphology, differentiation, survival, signaling responses, gene expression and proliferation that are found in vivo. Here, we propose to use UHMWPE as a material of the cellular matrix, the ultra-high molecular weight polyethylene. UHMWP is a bioinert substance, wich allows forming a system of open connected pores needed to provide cellular life conditions with supply of nutrients and oxygen as well as the removal of waste products, the possibility of intercellular communication, etc. As a result, the use of UHMWPE as a cellular matrix will allow to study the processes occurring in cells in the 3D environment.
ACKNOWLEDGEMENTS
Studies of the properties of polymers were carried out with the financial support of the Russian Science Foundation (Project No. 18-13-00145). The research of cellular models is carried out within the framework of the State Assignment of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences (No. 0090-2017-0019) and the Program of the Russian Academy of Sciences (Theme No. 48.8). The study was conducted using equipment of Center for Preclinical Trials and Center for collective use of IPAC RAS.
REFERENCES
- Chitnis, T. Weiner, H. L. (2017). CNS inflammation and neurodegeneration. Journal of Clinical Investigation, 127(10), 3577-3587. DOI
- Kempuraj, D., Thangavel, R., Natteru, P. A., Selvakumar, G. P., Saeed, D., Zahoor, H., Zaheer, S., Iyer, S. S., Zaheer, A. (2016). Neuroinflammation Induces Neurodegeneration. Journal of Neurology, Neurosurgery and Spine, 1(1). PMID: 28127589
- Edmondson, R., Broglie, J. J., Adcock, A. F., Yang, L. (2014). Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Development Technology, 12(4), 207-218. DOI
- Gurski, L. A., Petrelli, N. J., Jia, X., Farach-Carson, M. C. (2010). 3D Matrices for Anti-Cancer Drug Testing and Development. Oncology Issues, 25(1), 20-25. DOI
- Fang, Y. Eglen, R. M. (2017). Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discovery: Advancing Life Sciences R&D, 22(5), 456-472. DOI
- Birgersdotter, A., Sandberg, R., Ernberg, I. (2005). Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Seminars in Cancer Biology, 15(5), 405-412. DOI
- Alon, U., Barkai, N., Notterman, D. A., Gish, K., Ybarra, S., Mack, D., Levine, A. J. (1999). Broad patterns of gene expression revealed by clustering analysis of tumor and normal colon tissues probed by oligonucleotide arrays. Proc Natl Acad Sci U S A, 96(12), 6745-6750.
- Perou, C. M., Jeffrey, S. S., van de Rijn, M., Rees, C. A., Eisen, M. B., Ross, D. T., Pergamenschikov, A., Williams, C. F., Zhu, S. X., Lee, J. C., Lashkari, D., Shalon, D., Brown, P. O., Botstein, D. (1999). Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proceedings of the National Academy of Sciences of the United States of America, 96(16), 9212-9217.
- Alizadeh, A. A., Eisen, M. B., Davis, R. E., Ma, C., Lossos, I. S., Rosenwald, A., Boldrick, J. C., Sabet, H., Tran, T., Yu, X., Powell, J. I., Yang, L., Marti, G. E., Moore, T., Hudson, J., Jr., Lu, L., Lewis, D. B., Tibshirani, R., Sherlock, G., Chan, W. C., Greiner, T. C., Weisenburger, D. D., Armitage, J. O., Warnke, R., Levy, R., Wilson, W., Grever, M. R., Byrd, J. C., Botstein, D., Brown, P. O., Staudt, L. M. (2000). Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature, 403(6769), 503-511. DOI
- Ross, D. T., Scherf, U., Eisen, M. B., Perou, C. M., Rees, C., Spellman, P., Iyer, V., Jeffrey, S. S., Van de Rijn, M., Waltham, M., Pergamenschikov, A., Lee, J. C., Lashkari, D., Shalon, D., Myers, T. G., Weinstein, J. N., Botstein, D., Brown, P. O. (2000). Systematic variation in gene expression patterns in human cancer cell lines. Nature Genetics, 24(3), 227-235. DOI
- Virtanen, C., Ishikawa, Y., Honjoh, D., Kimura, M., Shimane, M., Miyoshi, T., Nomura, H., Jones, M. H. (2002). Integrated classification of lung tumors and cell lines by expression profiling. Proceedings of the National Academy of Sciences of the United States of America, 99(19), 12357-12362. DOI
- Schaner, M. E., Ross, D. T., Ciaravino, G., Sorlie, T., Troyanskaya, O., Diehn, M., Wang, Y. C., Duran, G. E., Sikic, T. L., Caldeira, S., Skomedal, H., Tu, I. P., Hernandez-Boussard, T., Johnson, S. W., O'Dwyer, P. J., Fero, M. J., Kristensen, G. B., Borresen-Dale, A. L., Hastie, T., Tibshirani, R., van de Rijn, M., Teng, N. N., Longacre, T. A., Botstein, D., Brown, P. O., Sikic, B. I. (2003). Gene expression patterns in ovarian carcinomas. Molecular Biology of the Cell, 14(11), 4376-4386. DOI
- Welsh, J. B., Sapinoso, L. M., Su, A. I., Kern, S. G., Wang-Rodriguez, J., Moskaluk, C. A., Frierson, H. F., Jr., Hampton, G. M. (2001). Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Research, 61(16), 5974-5978.
- Langhans, S. A. (2018). Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Frontiers in Pharmacology, 9, 6. DOI
- Maksimkin, A. V., Senatov, F. S., Anisimova, N. Y., Kiselevskiy, M. V., Zalepugin, D. Y., Chernyshova, I. V., Tilkunova, N. A., Kaloshkin, S. D. (2017). Multilayer porous UHMWPE scaffolds for bone defects replacement. Materials Science&Engineering. C, Materials for Biological Applications, 73, 366-372. DOI
- Maksimkin, A. V., Kharitonov, A. P., Mostovaya, K. S., Kaloshkin, S. D., Gorshenkov, M. V., Senatov, F. S., Chukov, D. I., Tcherdyntsev, V. V. (2016). Bulk oriented nanocomposites of ultrahigh molecular weight polyethylene reinforced with fluorinated multiwalled carbon nanotubes with nanofibrillar structure. Composites Part B: Engineering, 94, 292-298. DOI
- Maksimkin, A., Kaloshkin, S., Zadorozhnyy, M., Tcherdyntsev, V. (2014). Comparison of shape memory effect in UHMWPE for bulk and fiber state. Journal of Alloys and Compounds, 586, S214-S217. DOI
- Jakus, A. E., Secor, E. B., Rutz, A. L., Jordan, S. W., Hersam, M. C., Shah, R. N. (2015). Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. American Chemical Society Nano, 9(4), 4636-4648. DOI
- Pasca, A. M., Sloan, S. A., Clarke, L. E., Tian, Y., Makinson, C. D., Huber, N., Kim, C. H., Park, J. Y., O'Rourke, N. A., Nguyen, K. D., Smith, S. J., Huguenard, J. R., Geschwind, D. H., Barres, B. A., Pasca, S. P. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nature Methods, 12(7), 671-678. DOI
- Worthington, P., Drake, K. M., Li, Z., Napper, A. D., Pochan, D. J., Langhans, S. A. (2017). Beta-hairpin hydrogels as scaffolds for high-throughput drug discovery in three-dimensional cell culture. Analytical Biochemistry, 535, 25-34. DOI
- Lee, S. J., Zhu, W., Nowicki, M., Lee, G., Heo, D. N., Kim, J., Zuo, Y. Y., Zhang, L. G. (2018). 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. Journal of Neural Engineering, 15(1), 016018. DOI
- Wu, S., Duan, B., Lu, A., Wang, Y., Ye, Q., Zhang, L. (2017). Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates. Carbohydrate Polymer, 174, 830-840. DOI
- Shin, J., Choi, E. J., Cho, J. H., Cho, A. N., Jin, Y., Yang, K., Song, C., Cho, S. W. (2017). Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules, 18(10), 3060-3072. DOI
- Martin, C., Merino, S., Gonzalez-Dominguez, J. M., Rauti, R., Ballerini, L., Prato, M., Vazquez, E. (2017). Graphene Improves the Biocompatibility of Polyacrylamide Hydrogels: 3D Polymeric Scaffolds for Neuronal Growth. Scientific Reports, 7(1), 10942. DOI
- Struzyna, L. A., Adewole, D. O., Gordian-Velez, W. J., Grovola, M. R., Burrell, J. C., Katiyar, K. S., Petrov, D., Harris, J. P., Cullen, D. K. (2017). Anatomically Inspired Three-dimensional Micro-tissue Engineered Neural Networks for Nervous System Reconstruction, Modulation, and Modeling. Journal of Visualized Experiments. DOI: 10.3791/55609(123). DOI