The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Bioisosteric Analogues of Cinnamic Acid as Effective Neuroprotectors
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia,*e-mail: neganova83@mail.ru
2Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47 Leninsky av., Moscow, 119991 Russia
3Koltzov Institute of Developmental Biology, Russian Academy of Sciences, 26 Vavilova str., Moscow, 119334 Russia
4OOO Neurobotics, Directions 4922, 4-2, Southern Industrial Area, Zelenograd, 124498 Russia
4Ivanovo State University, School of Biology and Chemistry, Department of General Biology and Physiology, 136 Lenina av., Ivanovo, Russia
Key words: cinnamic acid; neuroprotectors; neurodegenerative diseases; mitochondria; antioxidants
DOI: 10.18097/BMCRM00052
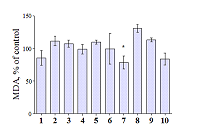
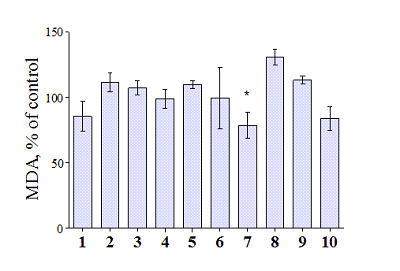
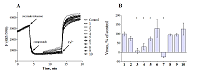
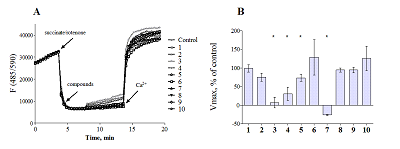
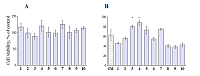
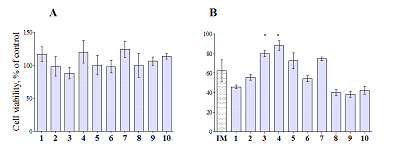
Compounds that act on mitochondrial functions are considered as promising drugs for the treatment of neurodegenerative diseases and age-related dementias. As a basis for the creation of such potential drugs, bioisosteric cinnamic acid analogs and polymethoxybenzene derivatives were selected. Derivatives of cinnamic acid have a wide range of biological activities, which can be important for drugs aimed at the treatment of neurodegenerative diseases, in particular Alzheimer's disease. In this work, the neuroprotective activity of bioisosteric cinnamic acid analogs and polymethoxybenzene derivatives was studied. Among the compounds studied, lead substances 3, 4, and 7 have been identified. These compounds show no intrinsic toxicity and have a neuroprotective effect on the cellular model of neurodegeneration associated with calcium stress. The mechanism of their cytoprotective activity is probably due to the influence on mitochondrial functions, because these compounds effectively suppress the calcium-induced process of mitochondrial permeability jump. In addition, one of the substances investigated (7/) has antioxidant properties, showing the ability to inhibit lipid peroxidation (LPO) of rat brain homogenate, which may be an additional mechanism of neuroprotective effect. The data obtained make it possible to recommend the investigated substances as a basis for the creation of effective neuroprotective drugs capable of influencing early stages of the development of neurodegenerative diseases.
|
CLOSE

|
Table 1.
Structures of test compounds.
|
ACKNOWLEDGEMENTS
The work was financially supported by RFBR grant No. 16-03-00079 and within the framework of the State Task of 2018 (topic No. 0090-2017-0019).
REFERENCES
- Leuner K., Muller W.E., Reichert A.S. (2012) From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol. Neurobiol, 46(1), 186–193. DOI
- Emerit J., Edeas M., Bricaire F. (2004) Neurodegenerative diseases and oxidative stress. Biomed Pharmacother, 58, 39-46. DOI
- Kumar, S., Arya, P., Mukherjee, C., Singh, B.K., Singh, N., Parmar, V.S., Prasad, A.K., Ghosh, B. (2005) Novel aromatic ester from Piper longum and its analogues inhibit expression of cell adhesion molecules on endothelial cells. Biochemistry, 44, 15944–15952. DOI
- Duchnowicz, P., Broncel, M., Podsedek, A., Koter-Michalak, M. (2012) Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur J Nutr, 51, 435–443. DOI
- Yabe, T., Hirahara, H., Harada, N., Ito, N., Nagal, T., Sangi, T., Yamada, H. (2010) Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience, 165, 515–524. DOI
- Amorati et al. (2013) Antioxidant Activity of Essential Oils. J. Agric. Food Chem., 61, 10835?10847. DOI
- Klochkov S.G., Neganova M.E., Afanas’eva S.V., & Shevtsova E.F. (2014) Synthesis and antioxidant activity of securinine derivatives. Pharmaceutical Chemistry Journal, 48(1), 15-17. DOI
- Bachurin, S.O., Shevtsova, E.P., Kireeva, E.G., Oxenkrug, G.F.& Sablin, S.O. (2003) Mitochondria as a target for neurotoxins and neuroprotective agents. Annals of the New Biochimica et Biophysica Acta, 1804(8), 1626–1634. DOI
- Akerman K.E., Wikstrom M.K. (1976) Safranine as a probe of the mitochondrial membrane potential. FEBS Lett, 68(2), 191-197. DOI
- Neganova M.E., Klochkov S.G., Afanasieva S.V., Serkova T.P., Chudinova E.S., Bachurin S.O., Reddy V.P., Aliev G., Shevtsova E.F. (2016) Neuroprotective effects of the securinine-analogues: identification of allomargaritarine as a lead compound. CNS Neurol. Disord. Drug Targets, 15(1), 102-107. DOI
- Niks M., Otto M. (1990) Towards an optimized MTT assay. J Immunol., 130(1), 149–151. DOI
- Lovell MA, Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. (1998) Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci., 158(1), 47-52. DOI
- Frederickson C.J., Koh J.Y., Bush A.I. (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci., 6(6), 449-62. DOI