The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Mitochondria are an Important Target in the Search for new Drugs for the Treatment of Alzheimer's Disease and Senile Dementia
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia,*e-mail: shevtsova@ipac.ac.ru
Key words: neurodegenerative diseases; mitochondria; calcium; mitochondrial permeability transition pore; Dimebon
DOI: 10.18097/BMCRM00058
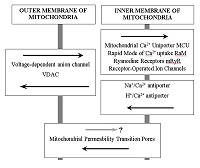
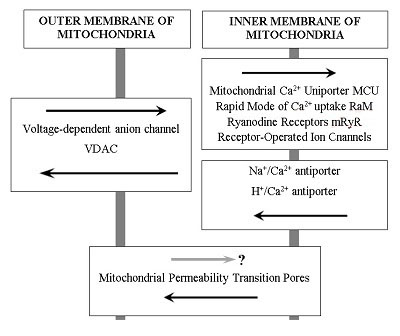
The review and summarizes own and literature data about the role of mitochondria as the important target in the search for drugs for the treatment of neurodegenerative diseases. Aging is a major risk factor for sporadic forms of various neurodegenerative diseases, including Alzheimer's disease. One of the most argued and currently accepted theories is the Mitochondrial Free Radical Theory of Aging. Mitochondrial hypotheses of the development of sporadic forms of neurodegenerative diseases particularly Alzheimer's disease, are closely connected with it. Impairments of mitochondrial functions lead to a decrease in their ability to regulate calcium homeostasis in the cell and to a decrease in the threshold for the induction of mitochondrial permeability transition (MPT) pores. MPT inhibitors can be considered as a promising approach to the treatment of neurodegenerative diseases, since these drugs can not only exhibit the properties of neuroprotectors, but also can provide normalization of synaptic activity due to increased calcium capacity of mitochondria. The review presents data on the number of MPT inhibitors, including endogenous compounds melatonin and N-acetylserotonin, their bioisosteric analogue Dimebon and a number of other compounds. The use of mitochondria as a basis for the formation of screening strategy for the search for compounds for the treatment of neurodegenerative diseases is of particular interest - both as a test of their potential toxicity, and as a basis for the creation of metabolic stimulants and drugs with neuroprotective and cognitive-stimulating effect.
ACKNOWLEDGEMENTS
This work was done in frames of the IPAC Research Program Framework 0090-2017-0019.
REFERENCES
- Qiu C., Kivipelto M., von Strauss E. (2009). Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci, 11(2), 111–128. DOI
- Eckert A., Schmitt K., Götz J. (2011). Mitochondrial dysfunction - the beginning of the end in Alzheimer’s disease? Separate and synergistic modes of tau and amyloid-β toxicity. Alzheimers. Res. Ther., 3(2), 15. DOI
- Swerdlow R.H., Burns J.M., Khan S.M. (2014). The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: Progress and Perspectives. Biochimica et biophysica acta, 1842(8), 1219-1231. DOI
- Caspersen C., Wang N., Yao J., Sosunov A., Chen X., Lustbader J.W., Xu H.W., Stern D., McKhann G., Yan S. Du. (2005). Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J., 19(14), 2040–2041. DOI
- Shevtzova E.F., Kireeva E.G., Bachurin S.O. (2001). Effect of beta-amyloid peptide fragment 25-35 on nonselective permeability of mitochondria. Bull Exp Biol Med., 132(6), 1173-1176. DOI
- Kumar A., Bodhinathan K., Foster T.C. (2009). Susceptibility to Calcium Dysregulation during Brain Aging. Front. Aging Neurosci., 1, 2. DOI
- Oh M.M., Oliveira F. a., Waters J., Disterhoft J.F. (2013). Altered Calcium Metabolism in Aging CA1 Hippocampal Pyramidal Neurons. J. Neurosci., 33(18), 7905–7911. DOI
- Mattson M.P. (2007). Calcium and neurodegeneration. Aging Cell., 6(3), 337–350. DOI
- Lopez J.R., Lyckman A., Oddo S., Laferla F.M., Querfurth H.W., Shtifman A. (2008). Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J. Neurochem., 105(1), 262–271. DOI 10.1111/j.1471-4159.2007.05135.x
- Crompton M. (1999). The mitochondrial permeability transition pore and its role in cell death. Biochem. J., 341, 233–249. DOI
- Peng T., Jou M. (2010). Oxidative stress caused by mitochondrial calcium overload., 1201,183–188. DOI 10.1111/j.1749-6632.2010.05634.x
- Rapizzi E., Pinton P., Szabadkai G., Wieckowski M.R., Vandecasteele G., Baird G., Tuft R.A., Fogarty K.E., Rizzuto R. (2002). Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol., 159(4), 613–624. DOI
- Giacomello M., Drago I., Pizzo P., Pozzan T. (2007). Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ., 14(7), 1267–1274. DOI
- Velikanov, G.A. (2013). Endoplasmic reticulum: Membrane contact sites Cell Tiss. Biol., 7, 504-511. https://doi.org/10.1134/S1990519X13060138
- Gingrich JR, Pelkey KA, Fam SR, Huang Y, Petralia RS, Wenthold RJ, Salter MW. (2004). Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci., 101, 6237–6241. DOI
- Shevtsova, E.P., Dubova, L.G., Kireeva, E.G., Bachurin, S.O. (2006). Mitochondria as the memantine target. European Neuropsychopharmacology, 16, 243-244. DOI
- Korde A.S., Maragos W.F. Identification of an N-methyl-D-aspartate receptor in isolated nervous system mitochondria. (2012). J Biol Chem., 287(42), 35192-35200. DOI
- Bernardi P. (2013). The mitochondrial permeability transition pore: a mystery solved? Front. Physiol. Frontiers, 4, 95. DOI
- Weeber E.J., Levy M., Sampson M.J., Anflous K., Armstrong D.L., Brown S.E., Sweatt J.D., Craigen W.J. (2002). The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J Biol Chem., 277(21), 18891-18897. DOI
- Skulachev V.P. (2012). Mitochondria-Targeted Antioxidants as Promising Drugs for Treatment of Age-Related Brain Diseases. J. Alzheimers Dis., 28 (2), 283-289. DOI
- Pavlov E., Zakharian E., Bladen C., Diao C.T.M., Grimbly C., Reusch R.N., French R.J. (2005). A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys. J., 88(4), 2614–2625. DOI 10.1529/biophysj.104.057281
- Abramov A.Y., Fraley C., Diao C.T., Winkfein R., Colicos M.A., Duchen M.R., French R.J., Pavlov E. (2007). Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc. Natl. Acad. Sci., 104(46), 18091–18096. DOI
- Kokoszka J.E., Waymire K.G., Levy S.E., Sligh J.E., Cai J., Jones D.P., MacGregor G.R., Wallace D.C. (2004). The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature, 427(6973), 461–465. DOI
- Varanyuwatana P., Halestrap A.P. (2012). The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion, 12, 120–125. DOI
- Papadopoulos V., Baraldi M., Guilarte T.R., Knudsen T.B., Lacapère J.-J., Lindemann P., Norenberg M.D., Nutt D., Weizman A., Zhang M.-R., Gavish M. (2006). Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci., 27(8), 402–409. DOI
- Roestenberg P., Manjeri G.R., Valsecchi F., Smeitink J. a M., Willems P.H.G.M., Koopman W.J.H. (2012). Pharmacological targeting of mitochondrial complex I deficiency: the cellular level and beyond. // Mitochondrion, 12(1), 57–65. DOI
- Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G.D., Petronilli V., Zoratti M., Szabó I., Lippe G., Bernardi P. (2013). ATP synthase dimers form the mitochondrial PTP. PNAS, 110(15), 5887-5892. DOI
- Bonora M., Bononi A., De Marchi E., Giorgi C., Lebiedzinska M., Marchi S., Patergnani S., Rimessi A., Suski J.M., Wojtala A., Wieckowski M.R., Kroemer G., Galluzzi L., Pinton P. (2013). Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle, 12(4), 674–683. DOI
- He J., Ford H.C., Carroll J., Ding S., Fearnley I.M., Walker J.E. (2017). Persistence of the mitochondrial permeability transition in the. absence of subunit c of human ATP synthase. Proc Natl Acad Sci U S A, 114, 3409-3414. DOI
- Kowaltowski A.J., Castilho R.F., Vercesi A.E. (2001). Mitochondrial permeability transition and oxidative stress. FEBS Lett., 495(1-2), 12–15. DOI
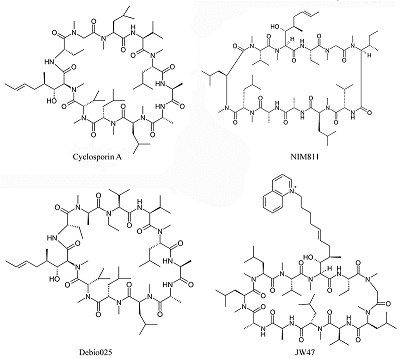
- Luciano M., Patrizia S., Annarita A. (2011). Cyclosporine A in Ullrich Congenital Muscular Dystrophy: Long-Term Results. Oxidative Medicine and Cellular Longevity, 2011, 1-10. DOI
- Nighoghossian N., Berthezène Y., Mechtouff L., Derex L., Cho TH., Ritzenthaler T., Rheims S., Chauveau F., Béjot Y., Jacquin A., Giroud M., Ricolfi F., Philippeau F., Lamy C., Turc G., Bodiguel E., Domigo V., Guiraud V., Mas J.L., Oppenheim C., Amarenco P., Cakmak S., Sevin-Allouet M., Guillon B., Desal H., Hosseini H., Sibon I., Mahagne M.H., Ong E., Mewton N., Ovize M. (2015). Cyclosporine in acute ischemic stroke. Neurology., 84(22), 2216-23. DOI
- Atkinson K., Britton K., Biggs J. (1984). Distribution and concentration of cyclosporin in human blood. Journal of Clinical Pathology., 37(10), 1167-1171. DOI
- Readnower R.D., Pandya J.D., McEwen M.L., Pauly J.R., Springer J.E., Sullivan P.G. (2011). Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J. Neurotrauma, 28(9), 1845–1853. DOI
- Korde A. S., Pettigrew L. C., Craddock S. D., Pocernich C. B., Waldmeier P. C., Maragos W. F. (2007). Protective Effects of NIM811 in Transient Focal Cerebral Ischemia Suggest Involvement of the Mitochondrial Permeability Transition. J. Neurotrauma, 24, 895−908. DOI
- Wissing E. R., Millay D. P., Vuagniaux G., Molkentin J. D. (2010). Debio-025 Is More Effective Than Prednisone in Reducing Muscular Pathology in Mdx Mice. Neuromuscul. Disord., 20, 753−760. DOI
- Warne J., Pryce G., Hill J.M., Shi X., Lennerås F., Puentes F., Kip M., Hilditch L., Walker P., Simone M.I., Chan A.W., Towers G.J., Coker A.R., Duchen M.R., Szabadkai G., Baker D., Selwood D.L. (2016). Selective Inhibition of the Mitochondrial Permeability Transition Pore Protects against Neurodegeneration in Experimental Multiple Sclerosis. J Biol Chem., 291(9), 4356-4373. DOI
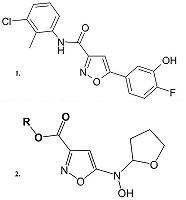
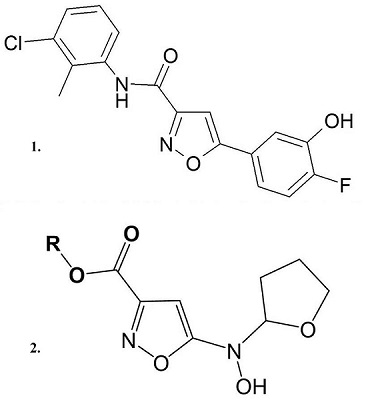
- Roy S., Šileikytė J., Schiavone M., Neuenswander B., Argenton F., Aubé J., Hedrick M.P., Chung T.D., Forte M.A., Bernardi P., Schoenen F.J. (2015). Discovery, Synthesis, and Optimization of Diarylisoxazole-3-carboxamides as Potent Inhibitors of the Mitochondrial Permeability Transition Pore. ChemMedChem., 10(10), 1655-71. DOI
- Averina E.B., Gracheva Y.A., Grishin Y.K., Radchenko E.V., Burmistrov V.V., Butov G.M., Neganova M.E., Serkova T.P., Redkozubova O.M., Shevtsova E.F., Milaeva E.R., Kuznetsova T.S., Zefirov N.S. (2016). Synthesis and biological evaluation of novel 5-hydroxylaminoisoxazole derivatives as lipoxygenase inhibitors and metabolism enhancing agents. Bioorganic & Medicinal Chemistry, 24(4), 712-720. DOI
- Smith R.A., Adlam V.J., Blaikie F.H., Manas A.R., Porteous C.M., James A.M., Ross M.F., Logan A., Cochemé H.M., Trnka J., Prime T.A., Abakumova I., Jones B.A., Filipovska A., Murphy M.P. (2008). Mitochondria-targeted antioxidants in the treatment of disease. Ann N Y Acad Sci., 1147, 105-111. DOI
- Rocha M., Hernandez-Mijares A., Garcia-Malpartida K., Bañuls C., Bellod L., Victor V.M. (2010). Mitochondria-targeted antioxidant peptides. Curr Pharm Des., 16(28), 3124-3131. DOI
- Srinivasan V., Spence D.W., Pandi-Perumal S.R., Brown G.M., Cardinali D.P. (2011). Melatonin in Mitochondrial Dysfunction and Related Disorders. International Journal of Alzheimer’s Disease, 2011, 1-16. DOI
- He H., Dong W., Huang F. (2010). Anti-amyloidogenic and anti-apoptotic role of melatonin in Alzheimer disease. Curr Neuropharmacol., 8(3), 211-217. DOI
- Pandi-Perumal S.R., BaHammam A.S., Brown G.M., Spence D.W., Bharti V.K., Kaur C., Hardeland R., Cardinali D.P. (2013). Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res., 23(3), 267-300. DOI
- Andrabi S.A., Sayeed I., Siemen D., Wolf G., Horn T.F. (2004). Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J., 18(7), 869-871. DOI
- Bachurin S., Oxenkrug G., Lermontova N., Afanasiev A., Beznosko B., Vankin G., Shevtsova E., Mukhina T., Serkova T. (2000). N-Acetyl-Serotonin, Melatonin and Their Derivatives Improve Cognition and Protect Against beta-Amyloid-induced Neurotoxicity. Annals of NY Aca of Sci., 890, 156-166. DOI
- Zhou H., Wang J., Jiang J., Stavrovskaya I.G., Li M., Li W., Wu Q., Zhang X., Luo C., Zhou S., Sirianni A.C., Sarkar S., Kristal B.S., Friedlander R.M., Wang X. (2014). N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J Neurosci., 34(8), 2967-2978. DOI
- Wang X., Figueroa B.E., Stavrovskaya I.G., Zhang Y., Sirianni A.C., Zhu S., Day A.L., Kristal B.S., Friedlander R.M. (2009). Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke, 40(5), 1877-85. DOI
- Bachurin S.O., Shevtsova E.P., Kireeva E.G., Oxenkrug G.F., Sablin S.O. (2003). Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci., 993, 334-344. DOI
- Millán-Plano Sergio, Eduardo Piedrafita, Francisco J. Miana-Mena , Lorena Fuentes-Broto. (2010). Melatonin and Structurally-Related Compounds Protect Synaptosomal Membranes from Free Radical Damage. J. Mol. Sci., 11, 312-328. DOI
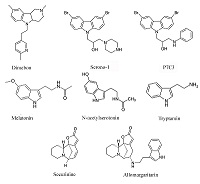
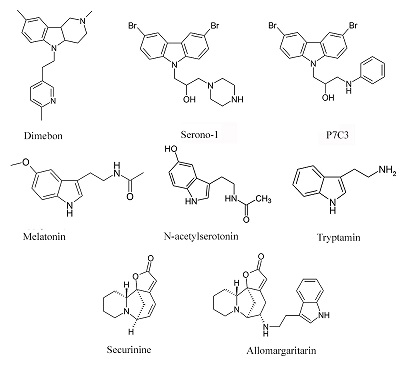
- Neganova M.E., Klochkov S.G., Afanasieva S.V., Chudinova E.S., Serkova T.P., Shevtsova E.F. (2014). Allomargaritarine as a basis for the creation of mitochondrial targeted potential neuroprotectors. Eur Neuropsychopharmacol., 24, 262. DOI
- Lin X., Jun-Tian Z. (2004). Neuroprotection by D-securinine against neurotoxicity induced by beta-amyloid (25-35). Neurol Res., 26(7), 792-796. DOI
- Raj D., Luczkiewicz M. (2008). Securinega suffruticosa. Fitoterapia, 79(6), 419-427. DOI
- Neganova M.E., Serkova T.P., Klochkov S.G., Afanasieva S.V., Shevtsova E.F., Bachurin S.O. (2011). Neuroprotective properties of Allomargaritarine, a Novel Tryptamine Derivative of the Natural Alkaloid Securinine Natural and Technical Sciences., 5, 86-90.
- Neganova M.E., Klochkov S.G., Afanasieva S.V., Serkova T.P., Chudinova E.S., Bachurin S.O., Reddy V.P., Aliev G., Shevtsova E.F. (2016). Neuroprotective effects of the securinine-analogues: identification of Allomargaritarine as a lead compound CNS & Neurological Disorders - Drug Targets, 15, 102-107. DOI : 10.2174/1871527314666150821111812
- Neganova M.E., Klochkov S.G., Petrova L.N., Shevtsova E.F., Afanasieva S.V., Chudinova E..S, Fisenko V.P., Bachurin S.O., Barreto G.E., Aliev G. (2017). Securinine Derivatives as Potential Anti-amyloid Therapeutic Approach. CNS Neurol Disord Drug Targets, 16(3), 351-355. DOI
- Neganova M.E., Blik V.A., Klochkov S.G., Chepurnova N.E., Shevtsova E.F. (2011). Investigation of the Antioxidant Characteristics of a New Tryptamine Derivative of Securinine and its Influence on Seizure Activity in the Brain in Experimental Epilepsy. Neurochemical Journal, 5, 208. DOI
- Kim do Y., Simeone K.A., Simeone T.A., Pandya J.D., Wilke J.C., Ahn Y., Geddes J.W., Sullivan P.G., Rho J.M. (2015). Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol., 78(1), 77-87. DOI
- Kudin A.P., Debska-Vielhaber G., Vielhaber S., Elger C.E., Kunz W.S. (2004). The mechanism of neuroprotection by topiramate in an animal model of epilepsy. Epilepsia, 45(12), 1478-1487. DOI
- Matveeva I.A. (1983). Action of dimebon on histamine receptors. Pharmakology and Toxicology, 46(4), 27–29. DOI
- Bachurin S.O., Shevtsova E.P., Kireeva E.G., Oxenkrug G.F., Sablin S.O. (2003). Mitochondria as a Target for Neurotoxins and Neuroprotective Agents. Ann. N.Y. Acad. Sci., 993, 334–344. DOI
- Shevtsova E.F., Vinogradova D.V., Kireeva E.G., V. Prakash Reddy, Aliev G., Bachurin S.O. (2015). Dimebon Attenuates the Rat-Brain Mitochondrial Permeabilization. Current Alzheimer Research, 11(5), 422-429. DOI
- Zhang S., Hedskog L., Petersen C.A., Winblad B., Ankarcrona M. (2010). Dimebon (latrepirdine) enhances mitochondrial function and protects neuronal cells from death. J Alzheimers Dis., 21(2), 389-402. DOI
- Bharadwaj P.R, Verdile G., Barr R.K., Gupta V., Steele J.W., Lachenmayer M.L., Yue Z., Ehrlich M.E., Petsko G., Ju S., Ringe D., Sankovich S.E., Caine J.M., Macreadie I.G., Gandy S., Martins R.N. (2012). Latrepirdine (dimebon) enhances autophagy and reduces intracellular GFP-Aβ42 levels in yeast. J Alzheimers Dis., 32(4), 949-67. DOI
- Eckert S.H., Eckmann J., Renner K., Eckert G..P, Leuner K., Muller W.E. (2012). Dimebon ameliorates amyloid-β induced impairments of mitochondrial form and function. J Alzheimers Dis., 31(1), 21-32. DOI
- Eckert S., Gaca J., Kolesova N. (2018). Mitochondrial Pharmacology of Dimebon (Latrepirdine) Calls for a New Look at its Possible Therapeutic Potential in Alzheimer’s Disease J. Aging Dis., 9(4), 729-744. DOI
- Bachurin, V. Grigoriev, E. Shevtsova, I. Koroleva, L. Dubova, E. Kireeva. (2007). Anti-aging properties of Dimebon: Relation to mitochondrial permeability inhibition, Experimental Gerontology, 42(1–2), 142-143. DOI
- Peters O.M., Shelkovnikova T., Tarasova T., Springe S., Kukharsky M.S., Smith G.A., Brooks S., Kozin S.A., Kotelevtsev Y., Bachurin S.O., Ninkina N., Buchman V.L. (2013). Chronic administration of Dimebon does not ameliorate amyloid-β pathology in 5xFAD transgenic mice. J Alzheimers Dis., 36(3), 589-596. DOI
- Peters O.M, Connor-Robson N., Sokolov V.B., Aksinenko A.Y., Kukharsky M.S., Bachurin S.O., Ninkina N., Buchman V.L. (2013). Chronic administration of dimebon ameliorates pathology in TauP301S transgenic mice. J Alzheimers Dis., 33(4), 1041-1049. DOI
- Bachurin S.O., Shelkovnikova T.A., Ustyugov A.A., Peters O., Khritankova I., Afanasieva M.A., Tarasova T.V., Alentov I.I., Buchman V.L., Ninkina N.N. (2012). Dimebon slows progression of proteinopathy in γ-synuclein transgenic mice. Neurotox Res., 22(1), 33-42. DOI
- Ustyugov A.A., Shelkovnikova T.A., Kokhan V.S., Khritankova I..V, Peters O., Buchman V.L., Bachurin S.O., Ninkina N.N. (2012). Dimebon reduces the levels of aggregated amyloidogenic protein forms in detergent-insoluble fractions in vivo. Bull Exp Biol Med., 152(6), 731-733. DOI
- Yamashita M., Nonaka T., Arai T., Kametani F., Buchman V.L., Ninkina N., Bachurin S.O., Akiyama H., Goedert M., Hasegawa M. (2009). Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett., 583(14), 2419-2424. DOI
- Khritankova I.V., Kukharskiy M.S., Lytkina O.A., Bachurin S.O., Shorning B.Y. (2012). Dimebon activates autophagosome components in human neuroblastoma SH-SY5Y cells. Dokl Biochem Biophys., 446, 251-253. DOI
- Pieper A.A, Xie S., Capota E., Estill S.J., Zhong J., Long J.M., Becker G.L., Huntington P., Goldman S.E., Shen C.H., Capota M., Britt J.K., Kotti T., Ure K., Brat D.J., Williams N.S., MacMillan K.S., Naidoo J., Melito L., Hsieh J., De Brabander J., Ready J.M., McKnight S.L. (2010). Discovery of a proneurogenic, neuroprotective chemical. Cell, 142(1), 39-51. DOI
- Hou Y., Mattson M.P., Cheng A. (2013). Permeability Transition Pore-Mediated Mitochondrial Superoxide Flashes Regulate Cortical Neural Progenitor Differentiation. PLoS ONE, 8(10), 76721. DOI
- Shin J.Y., Kong S.Y., Yoon H.J., Ann J., Lee J., Kim H.J. (2015). An Aminopropyl Carbazole Derivative Induces Neurogenesis by Increasing Final Cell Division in Neural Stem Cells. Biomol Ther (Seoul), 23(4), 313-319. DOI
- Wang G., Han T., Nijhawan D., Theodoropoulos P., Naidoo J., Yadavalli S., Mirzaei H., Pieper A.A., Ready J.M., McKnight S.L. (2014). P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell, 158(6), 1324-1334. DOI
- Wang S.N., Xu T.Y., Wang X., Guan Y.F., Zhang S.L., Wang P., Miao C.Y. (2016). Neuroprotective Efficacy of an Aminopropyl Carbazole Derivative P7C3-A20 in Ischemic Stroke. CNS Neurosci Ther, 22(9), 782-788. DOI
- Jiang B., Song L., Huang C., Zhang W. (2016). P7C3 Attenuates the Scopolamine-Induced Memory Impairments in C57BL/6J Mice. Neurochem Res., 41(5), 1010-1019. DOI
- Blaya M.O., Bramlett H.M., Naidoo J., Pieper A.A., Dietrich W.D. (2014). Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotrauma, 31(5), 476-486. DOI
- Kuharskiy M.S., Ovchinnikov R.K., Ustyugov A.A., Bachurin S.O. (2014). Molecular Aspects of Pathogenesis and Modern Appoaches to Pharmacological Correction of Alzheimer's Disease. Neurodegenerative Diseases: from the Genome to the Whole Organism, Ed. M.V.Ugryumov, Moscow, Scientific World, 2, 137-162.
- Bachurin S. (2015). Contemporary Approaches for Pharmacological Intervention of Abundant Neurodegenerative Disorders. J Nanomedine Biotherapeutic Discov, 5(2), 137. DOI