The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
MOLTRA-II. New three dimensional descriptors of the hydrogen bond
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: yarkov@ipac.ac.ru
Key words: 3-D descriptors; hydrogen bond descriptors
DOI: 10.18097/BMCRM00069
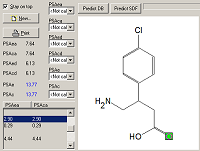
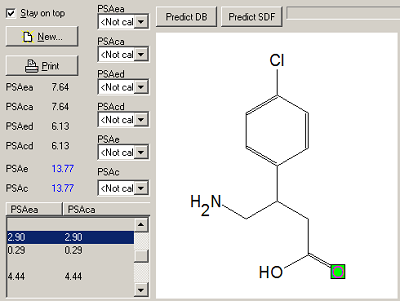
The program MOLTRA-II, created on the basis of the CHEmical Databases management system CHED, is supplemented by the possibility of calculating new descriptors based on the three-dimensional structure and hydrogen bond parameters. The program calculates PSA descriptors (Polar Surface Area), OSA (Optimal Structure Area) and energy of the hydrogen bond.
ACKNOWLEDGEMENTS
The work was performed within the framework of the state task for 2018 (the topic number 0090-2017-0020).
REFERENCES
- Raevsky, O.A., Grigoriev, V.Yu., Trepalin, S.V. (1999). HYBOT (Hydrogen Bond Thermodynamics). The certificate of official registration program for computer No. 990090.
- Trepalin, S.V., Yarkov, A.V., Razdolsky, A.N., Raevsky, O.A. (2014) MOLTRA-II. The new possibilities for calculation of molecular descriptors on the basis of inter atomic distances spectra. Modern high technologies, N12, 33-36.
- Trepalin, S.V., Yarkov, A.V. (2001). CheD: Chemical Database Compilation Tool, Internet Server, and client for SQL Servers. J. Chem. Inf. Comput. Sci., 41, 100-107. DOI
- Platts, J.A. (2000). Theoretical prediction of hydrogen bond basicity, Phys. Chem. Chem. Phys., 2 (14), 3115-3120. DOI
- Lamarche, O.,Platts, J.A. (2003). Complimentary nature of hydrogen bond basicity and acidity scale from electrostatic and atoms in molecules properties, Phys. Chem. Chem. Phys., 5 (4), 677-684. DOI
- Schwobel,J., Ebert,R.-U., Kuhne, R., Schuurmann, G. (2009). Prediction of intrinsic hydrogen bond acceptor strength of organic compounds by local molecular parameters, J. Chem. Inf. Model., 49(4), 956-962. DOI
- Hitchcock SA, Pennington LD (2006). Structure - Brain Exposure Relationships. J. Med. Chem. 49 (26): 7559–7583. DOI
- Raevsky, O.A., Skvortsov, V.S. (2005). Quantifying hydrogen bonding in QSAR and molecular modeling, SAR &QSAR in Enviromental Research, 16(3), 287-300. DOI
- Raevsky, O.A., Skvortsov, V.S. (2002). 3D hydrogen bond thermodynamics (HYBOT) potentials in molecular modelling, J.Comput. Aid Drug Design, 16(1), 1-10. DOI