The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Calculation and Analysis of Fractal Descriptors for Protein Amino Acids
in Various Conformational States
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: beng@ipac.ac.ru 2Department of Fundamental Physical and Chemical Engineering, Moscow State University, Moscow, 119991 Russia
Key words: amino acids; fractal descriptors; α-helix; β-sheet
DOI: 10.18097/BMCRM00070
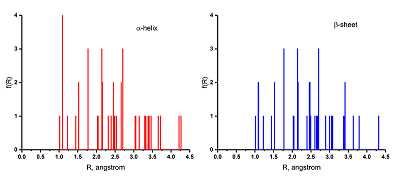
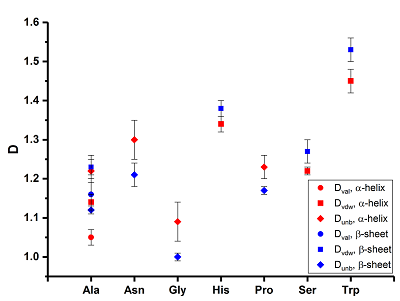
A series of 20 proteinogenic amino acids was studied. Four types of fractal descriptors for 2 conformational states are calculated: α-helix and 1-strand β-sheet. Based on the analysis of the results obtained, it is established that when the conformational state of the amino acids (α-helix→β-sheet) changes, significant changes in the fractal descriptor Dtot, in the calculation of which all the atoms of the molecule are used, are not observed. However, the more specific descriptors Dval, Dvdw and Dunb, which reflect the aggregate of valence-coupled, van der Waals contact and unbound atoms, respectively, are more sensitive to the conformational transition. The increase Dval, Dvdw and the decrease Dunb values were established for a series of 7 amino acids
|
CLOSE

|
Table 1.
Fractal descriptors (Dtot, Dval, Dvdw, Dunb and standard errors Δ) of amino acids (α-helix).
|
|
CLOSE

|
Table 2.
Fractal descriptors (Dtot, Dval, Dvdw, Dunb and standard errors Δ) of amino acids (β-sheet).
|
ACKNOWLEDGEMENTS
The work was performed within the framework of the State Task for 2018 (topic number 0090-2017-0020).
REFERENCES
- Anfinsen, C.B. (1973) Principles that govern the folding of protein chains. Science, 181(4096), 223-230. DOI
- Sweet, R.M., & Eisenberg, D. (1983) Correlation of sequence hydrophobicities measures similarity in three-dimensional protein structure. J. Mol. Biol., 171(4), 479-488. DOI
- Giuliani, A., Benigni, R., Zbilut, J.P., Webber, Jr., C.L., Sirabella, P., & Colosimo, A. (2002) Nonlinear signal analysis methods in the elucidation of protein sequence-structure relationships. Chem. Rev., 102(5), 1471-1492. DOI
- Huang, Y., Xiao, Y. (2003) Nonlinear deterministic structures and the randomness of protein sequences. Chaos, Solitons and Fractals, 17, 895-900. DOI
- Kanduc, D., Capone, G., Delfino, V.P., & Losa, G. (2010) The fractal dimension of protein information. Advanced Studies in Biology, 2(2), 53-62.
- Kornev, A.P., Taylor, S.S. (2017) Fractal nature of protein interior and its implications for protein function. Biophys. J., 112(3), 194A-195A. DOI
- Pavan, Y.S., Mitra, C.K. (2005) Fractal studies on the protein secondary structure elements. Ind. J. Biochem. Biophys., 42, 141-144.
- Yu, Z.G., Anh, V., & Lau, K.S. (2003) Multifractal and correlation analyses of protein sequences from complete genomes. Phys. Rev. E, 68, 021913. DOI
- Todoroff, N., Kunze, J., Schreuder, H., Hessler, G., Baringhaus, K.H., & Schneider, G. (2014) Fractal dimensions of macromolecular structures. Mol. Inf., 33, 588-596. DOI
- Andoyo, R., Lestari, V.D., Mardawati, E., & Nurhadi, B. (2018) Fractal dimension analysis of texture formation of whey protein-based foods. Int. J. Food Sci., 2018, article ID 7673259, 17 pages. DOI
- Grigor’ev, V.A., Raevskii, O.A. (2011) Fractal dimension of the interatomic distance histogram: new 3D descriptor of molecular structure. Russ. J. Gen. Chem., 81(3), 449-455. DOI
- HyperChem. Retrieved August 28, 2018, from http://www.hyper.com/
- Crownover, R.M. (1995) Introduction in fractals and chaos, Boston, Jones and Bartlett, 306.
- Grigorev, V.Yu., Grigoreva, L.D. (2016) Calculation and properties of fractal descriptors for C2–C9 alkanes. Moscow Univ. Chem. Bull., 71(3), 199-204. DOI
- Pande, V.S., Grosberg, A.Y., & Tanaka, T. (1994) Nonrandomness in protein sequences: evidence for a physically driven stage of evolution? Proc. Natl. Acad. Sci. USA, 91(26), 12972-12975.