The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
The HYBOT 3D MF Application and it Integration with CoMFA
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia,*e-mail: vladlen@ibmh.msk.su 2Institute of Biomedical Chemistry, 10 Pogodinskaya Street, Moscow, 119121, Russia
Key words: acetylcholine QSAR; CoMFA; HYBOT; molecular fields
DOI: 10.18097/BMCRM00073
The program "HYBOT 3D MF" based on the potential "6-8" modified by using HYBOT hydrogen bonding factors has been developed. An integration with QSAR and Advanced CoMFA" module of the SYBYL-X is performed by set of macros written in SPL. A full working version of the program is available at http://www.ibmc.msk.ru/HYBOT3D. For a number of sets of chemical compounds having pronounced differences in groups with the hydrogen bonding potential, the use of HYBOT 3D MF fields makes it possible to improve the predictive power of models created by the CoMFA technology.
|
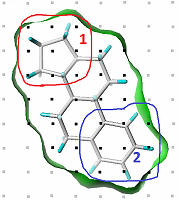
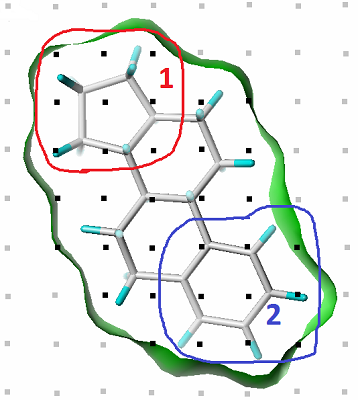
Figure 1.
An example of CoMFA steric field definition and grouping grid points as variables (components).
|
|
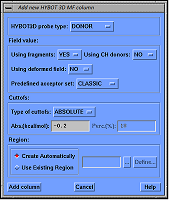
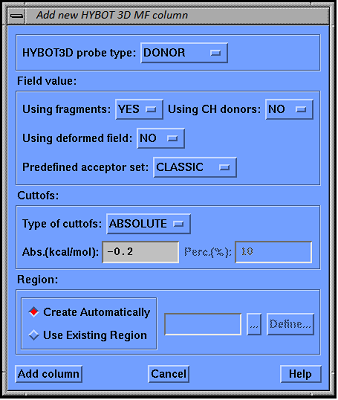
Figure 2.
The view of dialog for calculating parameters of donor or acceptor molecular field HYBOT 3D MF.
|
|
CLOSE

|
Table 1.
Statistical parameters of CoMFA models for various sets of arguments (example from SYBYL, table JACS, 21 steroids).
|
|
CLOSE

|
Table 2.
List of models, using CoMFA and CoMSIA for prediction inhibition of aldose reductase by flavonoid compounds (EC 1.1.1.21) [12].
|
ACKNOWLEDGEMENTS
The work was carried out within the framework of the state task for 2018 (topic number 0090-2017-0020).
REFERENCES
- Cramer, R. D., Patterson, D. E., & Bunce, J. D. (1988). Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. Journal of the American Chemical Society, 110(18), 5959-5967. DOI
- Ansari, S.M. and David S. Palmer, D.S. (2018). Comparative Molecular Field Analysis Using Molecular Integral Equation Theory. Journal of Chemical Information and Modeling 2018 58 (6), 1253-1265. DOI
- Goodford, P. J. (1985). A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. Journal of medicinal chemistry, 28(7), 849-857. DOI
- Cruciani, G., & Watson, K. A. (1994). Comparative molecular field analysis using GRID force-field and GOLPE variable selection methods in a study of inhibitors of glycogen phosphorylase b. Journal of medicinal chemistry, 37(16), 2589-2601. DOI
- Klebe, G., Abraham, U., & Mietzner, T. (1994). Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. Journal of medicinal chemistry, 37(24), 4130-4146. DOI
- Klebe, G., & Abraham, U. (1999). Comparative molecular similarity index analysis (CoMSIA) to study hydrogen-bonding properties and to score combinatorial libraries. Journal of computer-aided molecular design, 13(1), 1-10. DOI
- Zhokhova, N. I., Baskin, I. I., Bakhronov, D. K., Palyulin, V. A., & Zefirov, N. S. (2009, November). Method of continuous molecular fields in the search for quantitative structure-activity relationships. In Doklady Chemistry (Vol. 429, No. 1, p. 273). SP MAIK Nauka/Interperiodica.
- Boobbyer, D. N., Goodford, P. J., McWhinnie, P. M., & Wade, R. C. (1989). New hydrogen-bond potentials for use in determining energetically favorable binding sites on molecules of known structure. Journal of medicinal chemistry, 32(5), 1083-1094. DOI
- Raevsky, O. A., & Skvortsov, V. S. (2002). 3D hydrogen bond thermodynamics (HYBOT) potentials in molecular modelling. Journal of computer-aided molecular design, 16(1), 1-10. DOI
- Raevsky, O.A., Grigor’ev, V.Ju., Trepalin, S.V., HYBOT program package, Registration by Russian State Patent Agency No. 990090 of 26.02.99.
- SYBYL-X 2.1. Certara, Princeton, NJ, USA
- Caballero, J. (2010). 3D-QSAR (CoMFA and CoMSIA) and pharmacophore (GALAHAD) studies on the differential inhibition of aldose reductase by flavonoid compounds. Journal of Molecular Graphics and Modelling, 29(3), 363-371. DOI