Measuring Ciliary Neurotrophic Factor (CNTF) in Lacrimal Fluid Using Optimized Commercial ELISA
1Moscow Research and Clinical Center for Neuropsychiatry, Moscow Healthcare Department,
43 Donskaya street, Moscow, 115419 Russia; *e-mail: druzhkova.tatiana@mail.ru
2The S. Fyodorov Eye Microsurgery Federal State Institution,
Moscow, 59a Beskudnikovsky bulvar, Moscow, 127486 Russia
3Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences,
5A Butlerova street, Moscow, 117485 Russia
Keywords: ciliary neurotrophic factor; lacrimal fluid; sandwich enzyme immunoassay technique
DOI:10.18097/BMCRM00079
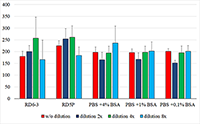
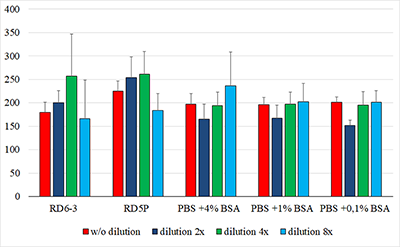
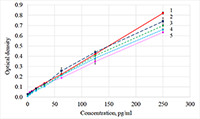
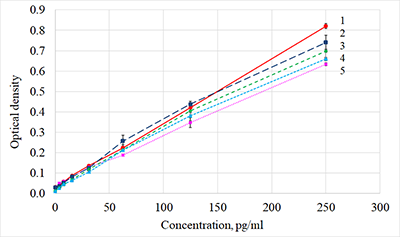
The concentration of ciliary neurotrophic factor (CNTF) was measured in lacrimal fluid (LF) using Human CNTF Quantikine ELISA kit («R&D Systems», USA) on a ChemWell 2910 automatic analyzer («Awareness Technology Inc.», USA). We initially attempted to use commercial kits, designed for serum and plasma CNTF detection, to quantify lacrimal CNTF. The results, however, were rarely above the minimum detection level of the kits, most likely due to matrix complexity and low concentrations of CNTF in diluted LF (LF had to be diluted because of the small volume of collected samples). The optimal sensitivity and the lowest background for the best minimum quantifiable value were determined empirically. Phosphate buffer solution containing 1% bovine serum albumin was selected as an optimal diluent for CNTF measurements in small fluid samples. A standard curve was produced using the calibrating solutions 0-250 pg/ml. Acid treatment of LF samples before the analysis allowed to increase the detectable concentration of the CNTF two-fold. The 1:3 dilution was selected based on the available volume of collected LF and a reasonable variation coefficient. The described protocol allowed to develop a sandwich ELISA optimized for lacrimal CNTF.
|
Figure 1. Calibration curves obtained by diluting the standard solution with different diluents.
1 - RD6-3, 2 - PBS +4% BSA, 3 - PBS +1% BSA, 4 - PBS +0,1% BSA, 5 - RD5P. |
|
CLOSE

|
Table 1.
Parameters of the enzyme immunoassay system using different diluents.
|
|
CLOSE

|
Table 2.
Lacrimal CNTF concentration (pg/ml) in samples with and without acid treatment at different dilutions.
|
|
CLOSE

|
Table 3.
Reproducibility of control measurements obtained on the 0 to 250 pg/ml calibration curve with PBS and 1% BSA diluent.
|
ACKNOWLEDGEMENTS
This study was supported by the Russian Foundation for Basic Research, grant № 18-015-00355.
REFERENCES
- Sleeman M.W., Anderson K.D., Lambert P.D., Yancopoulos G.D., Wiegand S.J. (2000). The ciliary neurotrophic factor and its receptor, CNTFR alpha. Pharm Acta Helv., 74 (2-3), 265-272. DOI
- Kang S.S., Keasey M.P., Cai J., Hagg T. (2012). Loss of neuron-astroglial interaction rapidly induces protective CNTF expression after stroke in mice. J Neurosci., 32(27), 9277–9287. DOI
- Purser M.J., Dalvi P.S. , Zi C. Wang Z.C., D.D., (2013). The Cytokine Ciliary Neurotrophic Factor (CNTF) Activates Hypothalamic Urocortin-Expressing Neurons Both In Vitro and In Vivo. PLoS One, 8 (4):e61616. DOI
- Valter K., Bisti S., Gargini C., Di Loreto S., Maccarone R., Cervetto L., Stone J. (2005). Time course of neurotrophic factor upregulation and retinal protection against light-induced damage after optic nerve section. Invest Ophthalmol Vis Sci., 46 (5), 1748-1754. DOI
- Miotke J.A., MacLennan A.J., Meyer R.L. J. (2007). Immunohistochemical localization of CNTFRalpha in adult mouse retina and optic nerve following intraorbital nerve crush: evidence for the axonal loss of a trophic factor receptor after injury. Comp. Neurol. 500, 384-400. DOI
- Ji J.Z., Elyaman W., Yip H.K., Lee V.W., Yick L.W., Hugon J., So K.F. (2004). CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci., 19(2), 265-272. DOI
- Liu X., Clark A.F., Wordinger R.J. (2007). Expression of ciliary neurotrophic factor (CNTF) and its tripartite receptor complex by cells of the human optic nerve head. Mol Vis., 13, 758-763.
- Sarup V., Patil K., Sharma S.C. (2004). Ciliary neurotrophic factor and its receptors are differentially expressed in the optic nerve transected adult rat retina. Brain Res., 1013(2), 152-158. DOI
- Wong F.S.Y., Tsang K.K., Lo A.C.Y. (2017). Delivery of therapeutics to posterior eye segment: cell-encapsulating systems. Neural Regen Res. 12(4), 576–577. DOI
- Zhang K., Hopkins J.J., Heier J.S., Birch D.G., Halperin L.S., Albini T.A., Brown D.M., Jaffe G.J., Tao W., Williams G.A. (2011). Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A., 108(15), 6241-6245. DOI
- Birch DG, Bennett LD, Duncan JL, Weleber RG, Pennesi ME (2016). Long-term follow-up of patients with retinitis pigmentosa receiving intraocular ciliary neurotrophic factor implants. Am J Ophthalmol. 170, 10-14. DOI
- Chowdhury U.R., Madden B.J., Charlesworth M.C., Fautsch M.P. (2010). Proteome Analysis of Human Aqueous Humor Invest Ophthalmol Vis Sci., 51, 4921-4931, DOI
- Okragly A.J., Haak-Frendscho M. (1997). An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp Neurol., 145(2 Pt 1):, 592-596. DOI
- Daniel W. Tholen, M.S., Chairholder Martin Kroll, M.D., FACB J. Rex Astles, Ph.D., FACB Albert L. Caffo, Ph.D. Thomas M. Happe, MT(ASCP) Jan Krouwer, Ph.D. Fred Lasky (2003) Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline, 23 (16).
- Lasky F.D., Boon D.J. , Eckfeldt J.H., Feldkamp C.S., Hassemer D., Sandy Krishnamurthy S., Long T.A., W.G. Miller, Naito H.K., PhD, Posner A. Clinical and Laboratory Standards Institute. Evaluation of Matrix Effects; Approved Guideline—Second Edition. CLSI document EP14-A2 EP14-A2, 25 (4).
- EP5-A2 Evaluation of Precision Performance of Quantitative Measurement Methods; Approved Guideline - Second Edition, 24, (25).
- Protocols for Determination of Limits of Detection and Limits of Quantitation; Approved Guideline EP17-A 24 (34).
- Kimata H. (2004). Passive smoking elevates neurotrophin levels in tears. Human & experimental toxicology, 23, 215-217. DOI
- Mandel A.L.; Ozdener H.; Utermohlen V. (2011). Brain-derived neurotrophic factor in human saliva: ELISA optimization and biological correlates. Journal of immunoassay & immunochemistry , 32, 18-30. DOI