Methods of Accelerated Prediction of The Shelf Life of Medical Polysaccharide-Based Hydrogels
1LLC «Coletex» , 4-6 non-residential premises, 21 Pavlovskaya str., Moscow,115093 Russia; *e-mail: koletex@list.ru
2Peoples' Friendship University of Russia, 3 Ordzhonikidze str., Moscow, 115419 Russia
Keywords:hydrogel materials; medical products; sodium alginate; accelerated aging; shelf life
DOI:10.18097/BMCRM00081
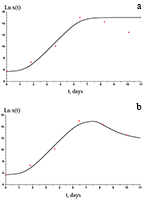
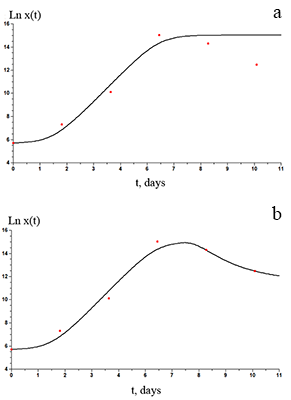
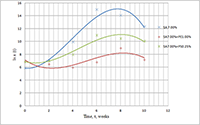
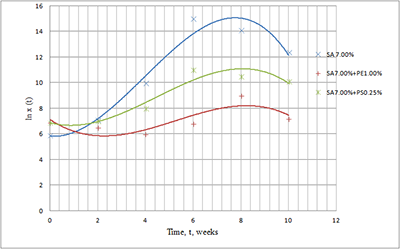
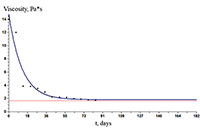
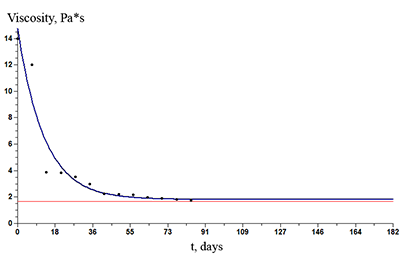
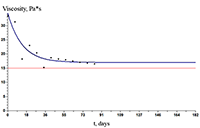
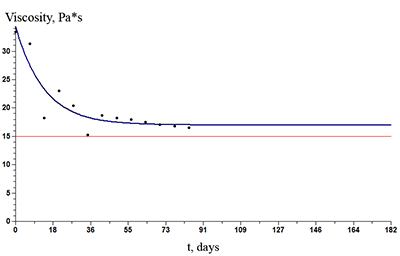
The paper describes an accelerated technique for determining the warranty period for the hydrogel-based polymer materials used for medical purposes. It deals with the technological production stages of hydrogel therapeutic materials «Kolegel» based on a polysaccharide of sodium alginate. On these stages deterioration of the material properties might lead to reduction of the shelf life of products (using natural raw materials, sterilization). The article introduces the ways to reduce this negative effect and subsequently increase the warranty period of the medical product by adding stabilizing additives into its composition. The method for determining the shelf life of sodium alginate hydrogel material depends on the added stabilizing additives (potassium sorbate and preservative based on 2-phenoxyethanol) in the «accelerated aging» mode; based on a mathematical description of two parallel processes occurring in hydrogels which are stored at elevated temperatures, namely: a change in the microbial contamination of hydrogels to the stage of final sterilization (using the Barany-Roberts model), a change in the dynamic viscosity after the conclusion of radiation sterilization of hydrogels (using a model based on the Arrhenius equation).
|
CLOSE

|
Table 1.
Terms of experimental storage depending on temperature.
|
SUPPLEMENTARY
Supplementary materials are available at http://dx.doi.org/10.18097/BMCRM00081
REFERENCES
- Vroman, I., & Tighzert, L. (2009). Biodegradable polymers. Materials 2: 307–344. DOI
- Boiko, A. V., Korytova, L. I., & Oltarzhevskaya, N.D.(2013). The directed delivery of medicines at treatment of oncological patients. M.: MK.
- Kildeeva, N. R., Vikhoreva, G.A., Galjbraykh, Hp, Mironov, A. V., Bonartseva, G.A., Perminov, P.A., & Romashova, A. N. (2006). Receiving the biodegraded porous films for use as wound coverings. Applied biochemistry and microbiology, 42(6), 716-720. DOI
- Kildeeva, N. R., Babak, V. G., Vikhoreva, G.A., Ageev, E. P., Golub, M.A., Galbraykh, L.S., & Merkovich, E. A. (2000). New approach to creation of materials with controlled release of medicinal substance. Bulletin of the Moscow University, 41(6), 423-425.
- Novikova, S. P., Bokeria, L. A., Bokeria, O.L., Salokhedinova, R. R., Nikolashina, L. N., Towns, A. Yu.... & Sivtsev, V. of Page (2015). The polymeric biodegraded film compositions and products on their basis for cardiovascular surgery. The bulletin NTsSSH of Bakulev's AN of the Russian Academy of Medical Science Cardiovascular diseases, 16(S3), 182-182.
- Sevostyanov, M.A., Nasakina, E. O., Baikin, Ampere-second., Leonov, A. V., Sergienko, K. V., Kaplan, M.A.... & Kolmakov, And. About (2016). Release kinetics in water solutions of an antibiotic of lincomycin from the biodegraded biopolymer membranes of medical application on the basis of chitosan of high density. Achievements of modern natural sciences, (12-2), 286-291.
- Shulanova, Zh. Zh. (2016). The prospects of application in surgery of biopolymer matriks on the basis of hyaluronic acid. Medical bulletin of Bashkortostan, 11(1 (61)), 135-138.
- Byrkina, T. S., Oltarzhevskaya, N. D., & Kolayeva, A. V. (2016). Ways of stabilization of microbiological and rheological indicators of medical depots materials "Kolegel". Vestnik of St. Petersburg State University of Technology and Design. Series 1. Natural and technical science, (3), 44-49.9.
- Kalashnikov, V. V., Naumova, L. A., Rabinkova, E. V., & Shishkova, O. V. (2012). Sterility of the products of medical appointment which underwent radiation in A.I. Burnazyan's FMBTs in 2007-2009. Medical radiology and radiation safety, 57(5), 66-71.
- Dai, Z., Ronholm, J., Tian, Y., Sethi, B., & Cao, X. (2016). Sterilization techniques for biodegradable scaffolds in tissue engineering applications. Journal of tissue engineering, 7, 2041731416648810. DOI
- Fedorova, A. V., Satalina, A. V., Fenin, A. A., Antropova, I. G., Oltarzhevskaya, N. D., Valuyeva, M.I., & Kolayeva, A. V. (2014). Destruction of medicinal substances at radiation sterilization. Butlerovsky messages, 38(4), 134-139.
- Sakayeva, I. V., Bunyatyan, N. D., Kovalyova, E. L., Sakanyan, E. I., Mitkina, L. I., Prokopov, I. A.... & Mitkina, Yu. V. (2013). Main approaches to studying of stability of medicines: domestic and international experience. Sheets of Scientific center of examination of means of medical application, (3).