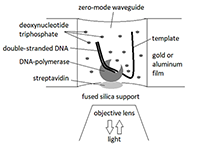
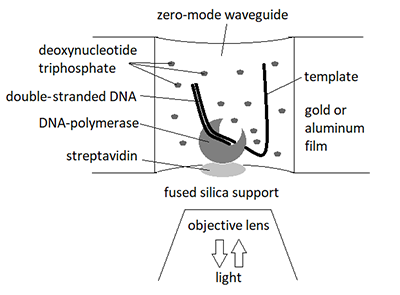
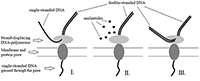
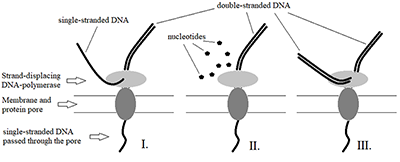
Prospects for the Use of Third Generation Sequencers for Quantitative Profiling of Transcriptome
1Institute of Biomedical Chemistry
10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: radkos@yandex.ru
2Adzhinomoto-Genetika
1 Dorozhny 1-st Road, Moscow, 117545 Russia
2Russian Scientific Center of Roentgenoradiology,
86 Profsoyuznaya str., Moscow, 117997 Russia
Keywords:third generation sequencing; transcriptome; quantitative profiling
DOI:10.18097/BMCRM00086
Transcriptome profiling is widely employed to analyze transcriptome dynamics when studying various biological processes at the cell and tissue levels. Unlike the second generation sequencers, which sequence relatively short fragments of nucleic acids, the third generation DNA/RNA sequencers developed by biotechnology companies “PacBio” and “Oxford Nanopore Technologies” allow one to sequence transcripts as single molecules and may be considered as potential molecular counters capable to measure the number of copies of each transcript with high throughput, sensitivity, and specificity. In the present review, the features of single molecule sequencing technologies offered by “PacBio” and “Oxford Nanopore Technologies” are considered alongside with their utility for transcriptome analysis, including the analysis of transcript isoforms. The prospects and limitations of the single molecule sequencing technology in application to quantitative transcriptome profiling are also discussed.
|
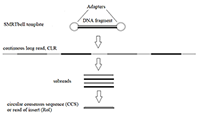
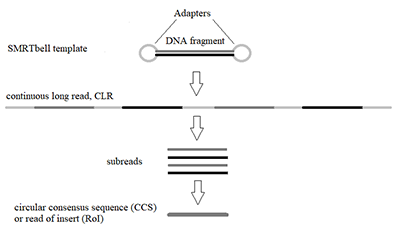
Figure 2.
Schematic representation of SMRTbell template and the stepwise treatments of raw reads during SMRT-sequencing.
|
|
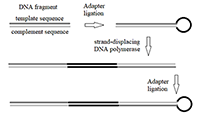
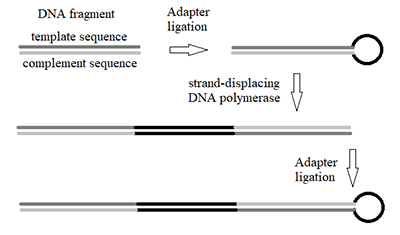
Figure 4.
Scheme of the design of double-stranded DNA fragment comprised of alternating «template» and «complement» sequences.y.
|
ACKNOWLEDGEMENTS
This work has been performed within the framework of the Fundamental Scientific Research Program of the State Academies of Sciences for 2013-2020.
REFERENCES
- Green, E. D., Watson, J. D., & Collins, F. S. (2015). Human Genome Project: Twenty-five years of big biology. Nature, 526(7571), 29-31. DOI
- Malone, J. H., & Oliver, B. (2011). Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biology, 9, 34. DOI
- Costa-Silva, J., Domingues, D., & Lopes, F. M. (2017). RNA-Seq differential expression analysis: An extended review and a software tool. PloS One, 12(12), e0190152. DOI
- Ambardar, S., Gupta, R., Trakroo, D., Lal, R., & Vakhlu, J. (2016). High Throughput Sequencing: An Overview of Sequencing Chemistry. Indian Journal of Microbiology, 56(4), 394-404. DOI
- Morey, M., Fernandez-Marmiesse, A., Castineiras, D., Fraga, J. M., Couce, M. L., & Cocho, J. A. (2013). A glimpse into past, present, and future DNA sequencing. Molecular Genetics and Metabolism, 110(1-2), 3-24. DOI
- Choi, S. C. (2016). On the study of microbial transcriptomes using second- and third-generation sequencing technologies. Journal of Microbiology, 54(8), 527-536. DOI
- Pillai, S., Gopalan, V., & Lam, A. K. (2017). Review of sequencing platforms and their applications in phaeochromocytoma and paragangliomas. Critical Reviews in Oncology/Hematology, 116, 58-67. DOI
- Schadt, E. E., Turner, S., & Kasarskis, A. (2010). A window into third-generation sequencing. Human Molecular Genetics, 19(R2), R227-240. DOI
- Ashton, P. M., Nair, S., Dallman, T., Rubino, S., Rabsch, W., Mwaigwisya, S., Wain, J., & O'Grady, J. (2015). MinION nanopore sequencing identifies the position and structure of a bacterial antibiotic resistance island. Nature Biotechnology, 33(3), 296-300. DOI
- Laver, T., Harrison, J., O'Neill, P. A., Moore, K., Farbos, A., Paszkiewicz, K., & Studholme, D. J. (2015). Assessing the performance of the Oxford Nanopore Technologies MinION. Biomolecular Detection and Quantification, 3, 1-8. DOI
- Eid, J., Fehr, A., Gray, J., Luong, K., Lyle, J., Otto, G., Peluso, P., Rank, D., Baybayan, P., Bettman, B., Bibillo, A., Bjornson, K., Chaudhuri, B., Christians, F., Cicero, R., Clark, S., Dalal, R., Dewinter, A., Dixon, J., Foquet, M., Gaertner, A., Hardenbol, P., Heiner, C., Hester, K., Holden, D., Kearns, G., Kong, X., Kuse, R., Lacroix, Y., Lin, S., Lundquist, P., Ma, C., Marks, P., Maxham, M., Murphy, D., Park, I., Pham, T., Phillips, M., Roy, J., Sebra, R., Shen, G., Sorenson, J., Tomaney, A., Travers, K., Trulson, M., Vieceli, J., Wegener, J., Wu, D., Yang, A., Zaccarin, D., Zhao, P., Zhong, F., Korlach, J., & Turner, S. (2009). Real-time DNA sequencing from single polymerase molecules. Science, 323(5910), 133-138. DOI
- Korlach, J., Bjornson, K. P., Chaudhuri, B. P., Cicero, R. L., Flusberg, B. A., Gray, J. J., Holden, D., Saxena, R., Wegener, J., & Turner, S. W. (2010). Real-time DNA sequencing from single polymerase molecules. Methods in Enzymology, 472, 431-455. DOI
- Beckett, D., Kovaleva, E., & Schatz, P. J. (1999). A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Science, 8(4), 921-929. DOI
- Lundquist, P. M., Zhong, C. F., Zhao, P., Tomaney, A. B., Peluso, P. S., Dixon, J., Bettman, B., Lacroix, Y., Kwo, D. P., McCullough, E., Maxham, M., Hester, K., McNitt, P., Grey, D. M., Henriquez, C., Foquet, M., Turner, S. W., & Zaccarin, D. (2008). Parallel confocal detection of single molecules in real time. Optics Letters, 33(9), 1026-1028
- Travers, K. J., Chin, C. S., Rank, D. R., Eid, J. S., & Turner, S. W. (2010). A flexible and efficient template format for circular consensus sequencing and SNP detection. Nucleic Acids Research, 38(15), e159. DOI
- An, D., Cao, H. X., Li, C., Humbeck, K., & Wang, W. (2018). Isoform Sequencing and State-of-Art Applications for Unravelling Complexity of Plant Transcriptomes. Genes, 9(1). DOI
- https://github.com/PacificBiosciences/IsoSeq_SA3nUP, last accessed on December 1, 2018. DOI
- Nakano, K., Shiroma, A., Shimoji, M., Tamotsu, H., Ashimine, N., Ohki, S., Shinzato, M., Minami, M., Nakanishi, T., Teruya, K., Satou, K., & Hirano, T. (2017). Advantages of genome sequencing by long-read sequencer using SMRT technology in medical area. Human Cell, 30(3), 149-161. DOI
- Mardis, E. R. (2013). Next-generation sequencing platforms. Annual Review of Analytical Chemistry, 6, 287-303. DOI
- Rhoads, A., & Au, K. F. (2015). PacBio Sequencing and Its Applications. Genomics, Proteomics & Bioinformatics, 13(5), 278-289. DOI
- Vilfan, I. D., Tsai, Y. C., Clark, T. A., Wegener, J., Dai, Q., Yi, C., Pan, T., Turner, S. W., & Korlach, J. (2013). Analysis of RNA base modification and structural rearrangement by single-molecule real-time detection of reverse transcription. Journal of Nanobiotechnology, 11, 8. DOI
- Kasianowicz, J. J., Brandin, E., Branton, D., & Deamer, D. W. (1996). Characterization of individual polynucleotide molecules using a membrane channel. Proceedings of the National Academy of Sciences of the United States of America, 93(24), 13770-13773.
- Akeson, M., Branton, D., Kasianowicz, J. J., Brandin, E., & Deamer, D. W. (1999). Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophysical Journal, 77(6), 3227-3233. DOI
- Bayley, H. (2015). Nanopore sequencing: from imagination to reality. Clinical Chemistry, 61(1), 25-31. DOI
- Lieberman, K. R., Cherf, G. M., Doody, M. J., Olasagasti, F., Kolodji, Y., & Akeson, M. (2010). Processive replication of single DNA molecules in a nanopore catalyzed by phi29 DNA polymerase. Journal of the American Chemical Society, 132(50), 17961-17972. DOI
- Cherf, G. M., Lieberman, K. R., Rashid, H., Lam, C. E., Karplus, K., & Akeson, M. (2012). Automated forward and reverse ratcheting of DNA in a nanopore at 5-A precision. Nature Biotechnology, 30(4), 344-348. DOI
- Manrao, E. A., Derrington, I. M., Pavlenok, M., Niederweis, M., & Gundlach, J. H. (2011). Nucleotide discrimination with DNA immobilized in the MspA nanopore. PloS One, 6(10), e25723. DOI
- Manrao, E. A., Derrington, I. M., Laszlo, A. H., Langford, K. W., Hopper, M. K., Gillgren, N., Pavlenok, M., Niederweis, M., & Gundlach, J. H. (2012). Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nature Biotechnology, 30(4), 349-353. DOI
- Laszlo, A. H., Derrington, I. M., Ross, B. C., Brinkerhoff, H., Adey, A., Nova, I. C., Craig, J. M., Langford, K. W., Samson, J. M., Daza, R., Doering, K., Shendure, J., & Gundlach, J. H. (2014). Decoding long nanopore sequencing reads of natural DNA. Nature Biotechnology, 32(8), 829-833. DOI
- US Patent N 9447152 B2.
- US Patent N 9751915 B2.
- US Patent Application N 2015/0068904 A1.
- US Patent N 9758823 B2.
- US Patent Application N 2015/0065354 A1.
- US Patent Application N 2015/0152492 A1.
- US Patent Application N 2016/0162634 A1.
- US Patent Application N 2015/0014160 A1.
- US Patent N 10036065 B2.
- US Patent N 9651519 B2.
- Garalde, D. R., Snell, E. A., Jachimowicz, D., Sipos, B., Lloyd, J. H., Bruce, M., Pantic, N., Admassu, T., James, P., Warland, A., Jordan, M., Ciccone, J., Serra, S., Keenan, J., Martin, S., McNeill, L., Wallace, E. J., Jayasinghe, L., Wright, C., Blasco, J., Young, S., Brocklebank, D., Juul, S., Clarke, J., Heron, A. J., & Turner, D. J. (2018). Highly parallel direct RNA sequencing on an array of nanopores. Nature Methods, 15(3), 201-206. DOI
- Keller, M. W., Rambo-Martin, B. L., Wilson, M. M., Ridenour, C. A., Shepard, S. S., Stark, T. J., Neuhaus, E. B., Dugan, V. G., Wentworth, D. E., & Barnes, J. R. (2018). Direct RNA Sequencing of the Coding Complete Influenza A Virus Genome. Scientific Reports, 8(1), 14408. DOI
- Seki, M., Katsumata, E., Suzuki, A., Sereewattanawoot, S., Sakamoto, Y., Mizushima-Sugano, J., Sugano, S., Kohno, T., Frith, M. C., Tsuchihara, K., & Suzuki, Y. (2018). Evaluation and application of RNA-Seq by MinION. DNA Research, DOI
- Jenjaroenpun, P., Wongsurawat, T., Pereira, R., Patumcharoenpol, P., Ussery, D. W., Nielsen, J., & Nookaew, I. (2018). Complete genomic and transcriptional landscape analysis using third-generation sequencing: a case study of Saccharomyces cerevisiae CEN.PK113-7D. Nucleic Acids Research, 46(7), e38. DOI
- Moldovan, N., Tombacz, D., Szucs, A., Csabai, Z., Balazs, Z., Kis, E., Molnar, J., & Boldogkoi, Z. (2018). Third-generation Sequencing Reveals Extensive Polycistronism and Transcriptional Overlapping in a Baculovirus. Scientific Reports, 8(1), 8604. DOI
- Jain, M., Tyson, J. R., Loose, M., Ip, C. L. C., Eccles, D. A., O'Grady, J., Malla, S., Leggett, R. M., Wallerman, O., Jansen, H. J., Zalunin, V., Birney, E., Brown, B. L., Snutch, T. P., Olsen, H. E., Min, I. O. N. A., & Reference, C. (2017). MinION Analysis and Reference Consortium: Phase 2 data release and analysis of R9.0 chemistry. F1000Research, 6, 760. DOI
- Butt, S. L., Taylor, T. L., Volkening, J. D., Dimitrov, K. M., Williams-Coplin, D., Lahmers, K. K., Miller, P. J., Rana, A. M., Suarez, D. L., Afonso, C. L., & Stanton, J. B. (2018). Rapid virulence prediction and identification of Newcastle disease virus genotypes using third-generation sequencing. Virology Journal, 15(1), 179. DOI
- Rames, E., & Macdonald, J. (2018). Evaluation of MinION nanopore sequencing for rapid enterovirus genotyping. Virus Research, 252, 8-12. DOI
- Li, C., Chng, K. R., Boey, E. J., Ng, A. H., Wilm, A., & Nagarajan, N. (2016). INC-Seq: accurate single molecule reads using nanopore sequencing. GigaScience, 5(1), 34. DOI
- Batovska, J., Lynch, S. E., Rodoni, B. C., Sawbridge, T. I., & Cogan, N. O. (2017). Metagenomic arbovirus detection using MinION nanopore sequencing. Journal of Virological Methods, 249, 79-84. DOI
- Au, K. F., Sebastiano, V., Afshar, P. T., Durruthy, J. D., Lee, L., Williams, B. A., van Bakel, H., Schadt, E. E., Reijo-Pera, R. A., Underwood, J. G., & Wong, W. H. (2013). Characterization of the human ESC transcriptome by hybrid sequencing. Proceedings of the National Academy of Sciences of the United States of America, 110(50), E4821-4830. DOI
- Steijger, T., Abril, J. F., Engstrom, P. G., Kokocinski, F., Consortium, R., Hubbard, T. J., Guigo, R., Harrow, J., & Bertone, P. (2013). Assessment of transcript reconstruction methods for RNA-seq. Nature Methods, 10(12), 1177-1184. DOI
- Au, K. F., Underwood, J. G., Lee, L., & Wong, W. H. (2012). Improving PacBio long read accuracy by short read alignment. PloS One, 7(10), e46679. DOI
- Hu, R., Sun, G., & Sun, X. (2016). LSCplus: a fast solution for improving long read accuracy by short read alignment. BMC Bioinformatics, 17(1), 451. DOI
- Salmela, L., & Rivals, E. (2014). LoRDEC: accurate and efficient long read error correction. Bioinformatics, 30(24), 3506-3514. DOI
- Hackl, T., Hedrich, R., Schultz, J., & Forster, F. (2014). proovread: large-scale high-accuracy PacBio correction through iterative short read consensus. Bioinformatics, 30(21), 3004-3011. DOI
- Chao, Q., Gao, Z. F., Zhang, D., Zhao, B. G., Dong, F. Q., Fu, C. X., Liu, L. J., & Wang, B. C. (2018). The developmental dynamics of the Populus stem transcriptome. Plant Biotechnology Journal. DOI
- Filichkin, S. A., Hamilton, M., Dharmawardhana, P. D., Singh, S. K., Sullivan, C., Ben-Hur, A., Reddy, A. S. N., & Jaiswal, P. (2018). Abiotic Stresses Modulate Landscape of Poplar Transcriptome via Alternative Splicing, Differential Intron Retention, and Isoform Ratio Switching. Frontiers in Plant Science, 9, 5. DOI
- Piriyapongsa, J., Kaewprommal, P., Vaiwsri, S., Anuntakarun, S., Wirojsirasak, W., Punpee, P., Klomsa-Ard, P., Shaw, P. J., Pootakham, W., Yoocha, T., Sangsrakru, D., Tangphatsornruang, S., Tongsima, S., & Tragoonrung, S. (2018). Uncovering full-length transcript isoforms of sugarcane cultivar Khon Kaen 3 using single-molecule long-read sequencing. PeerJ, 6, e5818. DOI
- Zhang, G., Sun, M., Wang, J., Lei, M., Li, C., Zhao, D., Huang, J., Li, W., Li, S., Li, J., Yang, J., Luo, Y., Hu, S., & Zhang, B. (2018). PacBio full-length cDNA sequencing integrated with RNA-seq reads drastically improves the discovery of splicing transcripts in rice. The Plant Journal, DOI
- Zhu, J., Wang, X., Xu, Q., Zhao, S., Tai, Y., & Wei, C. (2018). Global dissection of alternative splicing uncovers transcriptional diversity in tissues and associates with the flavonoid pathway in tea plant (Camellia sinensis). BMC Plant Biology, 18(1), 266. DOI
- Kim, J. Y., Lim, H. Y., Shin, S. E., Cha, H. K., Seo, J. H., Kim, S. K., Park, S. H., & Son, G. H. (2018). Comprehensive transcriptome analysis of Sarcophaga peregrina, a forensically important fly species. Scientific Data, 5, 180220. DOI
- Zhu, C., Li, X., & Zheng, J. (2018). Transcriptome profiling using Illumina- and SMRT-based RNA-seq of hot pepper for in-depth understanding of genes involved in CMV infection. Gene, 666, 123-133. DOI
- Singh, N., Sahu, D. K., Chowdhry, R., Mishra, A., Goel, M. M., Faheem, M., Srivastava, C., Ojha, B. K., Gupta, D. K., & Kant, R. (2016). IsoSeq analysis and functional annotation of the infratentorial ependymoma tumor tissue on PacBio RSII platform. Meta Gene, 7, 70-75. DOI
- Sahlin, K., Tomaszkiewicz, M., Makova, K. D., & Medvedev, P. (2018). Deciphering highly similar multigene family transcripts from Iso-Seq data with IsoCon. Nature Communications, 9(1), 4601. DOI
- Balazs, Z., Tombacz, D., Szucs, A., Snyder, M., & Boldogkoi, Z. (2018). Dual Platform Long-Read RNA-Sequencing Dataset of the Human Cytomegalovirus Lytic Transcriptome. Frontiers in Genetics, 9, 432. DOI
- Workman, R. E., Myrka, A. M., Wong, G. W., Tseng, E., Welch, K. C., Jr., & Timp, W. (2018). Single-molecule, full-length transcript sequencing provides insight into the extreme metabolism of the ruby-throated hummingbird Archilochus colubris. GigaScience, 7(3), 1-12. DOI
- Yi, S., Zhou, X., Li, J., Zhang, M., & Luo, S. (2018). Full-length transcriptome of Misgurnus anguillicaudatus provides insights into evolution of genus Misgurnus. Scientific Reports, 8(1), 11699. DOI
- Nudelman, G., Frasca, A., Kent, B., Sadler, K. C., Sealfon, S. C., Walsh, M. J., & Zaslavsky, E. (2018). High resolution annotation of zebrafish transcriptome using long-read sequencing. Genome Research, 28(9), 1415-1425. DOI
- Dong, L., Liu, H., Zhang, J., Yang, S., Kong, G., Chu, J. S., Chen, N., & Wang, D. (2015). Single-molecule real-time transcript sequencing facilitates common wheat genome annotation and grain transcriptome research. BMC Genomics, 16, 1039. DOI
- Wang, T., Wang, H., Cai, D., Gao, Y., Zhang, H., Wang, Y., Lin, C., Ma, L., & Gu, L. (2017). Comprehensive profiling of rhizome-associated alternative splicing and alternative polyadenylation in moso bamboo (Phyllostachys edulis). The Plant Journal, 91(4), 684-699. DOI
- Abdel-Ghany, S. E., Hamilton, M., Jacobi, J. L., Ngam, P., Devitt, N., Schilkey, F., Ben-Hur, A., & Reddy, A. S. (2016). A survey of the sorghum transcriptome using single-molecule long reads. Nature Communications, 7, 11706. DOI
- Zhang, S. J., Wang, C., Yan, S., Fu, A., Luan, X., Li, Y., Sunny Shen, Q., Zhong, X., Chen, J. Y., Wang, X., Chin-Ming Tan, B., He, A., & Li, C. Y. (2017). Isoform Evolution in Primates through Independent Combination of Alternative RNA Processing Events. Molecular Biology and Evolution, 34(10), 2453-2468. DOI
- Liu, X., Mei, W., Soltis, P. S., Soltis, D. E., & Barbazuk, W. B. (2017). Detecting alternatively spliced transcript isoforms from single-molecule long-read sequences without a reference genome. Molecular Ecology Resources, 17(6), 1243-1256. DOI
- Wu, T. D., & Watanabe, C. K. (2005). GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics, 21(9), 1859-1875. DOI
- Gordon, S. P., Tseng, E., Salamov, A., Zhang, J., Meng, X., Zhao, Z., Kang, D., Underwood, J., Grigoriev, I. V., Figueroa, M., Schilling, J. S., Chen, F., & Wang, Z. (2015). Widespread Polycistronic Transcripts in Fungi Revealed by Single-Molecule mRNA Sequencing. PloS One, 10(7), e0132628. DOI
- Tardaguila, M., de la Fuente, L., Marti, C., Pereira, C., Pardo-Palacios, F. J., Del Risco, H., Ferrell, M., Mellado, M., Macchietto, M., Verheggen, K., Edelmann, M., Ezkurdia, I., Vazquez, J., Tress, M., Mortazavi, A., Martens, L., Rodriguez-Navarro, S., Moreno-Manzano, V., & Conesa, A. (2018). SQANTI: extensive characterization of long-read transcript sequences for quality control in full-length transcriptome identification and quantification. Genome Research. DOI
- Deng, Y., Zheng, H., Yan, Z., Liao, D., Li, C., Zhou, J., & Liao, H. (2018). Full-Length Transcriptome Survey and Expression Analysis of Cassia obtusifolia to Discover Putative Genes Related to Aurantio-Obtusin Biosynthesis, Seed Formation and Development, and Stress Response. International Journal of Molecular Sciences, 19(9). DOI
- Ren, P., Meng, Y., Li, B., Ma, X., Si, E., Lai, Y., Wang, J., Yao, L., Yang, K., Shang, X., & Wang, H. (2018). Molecular Mechanisms of Acclimatization to Phosphorus Starvation and Recovery Underlying Full-Length Transcriptome Profiling in Barley (Hordeum vulgare L.). Frontiers in Plant Science, 9, 500. DOI
- Xu, Z., Peters, R. J., Weirather, J., Luo, H., Liao, B., Zhang, X., Zhu, Y., Ji, A., Zhang, B., Hu, S., Au, K. F., Song, J., & Chen, S. (2015). Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. The Plant Journal, 82(6), 951-961. DOI
- Cao, H., Lai, Y., Bougouffa, S., Xu, Z., & Yan, A. (2017). Comparative genome and transcriptome analysis reveals distinctive surface characteristics and unique physiological potentials of Pseudomonas aeruginosa ATCC 27853. BMC Genomics, 18(1), 459. DOI
- Ning, G., Cheng, X., Luo, P., Liang, F., Wang, Z., Yu, G., Li, X., Wang, D., & Bao, M. (2017). Hybrid sequencing and map finding (HySeMaFi): optional strategies for extensively deciphering gene splicing and expression in organisms without reference genome. Scientific Reports, 7, 43793. DOI
- Tombacz, D., Balazs, Z., Csabai, Z., Moldovan, N., Szucs, A., Sharon, D., Snyder, M., & Boldogkoi, Z. (2017). Characterization of the Dynamic Transcriptome of a Herpesvirus with Long-read Single Molecule Real-Time Sequencing. Scientific Reports, 7, 43751. DOI
- Young, G. R., Terry, S. N., Manganaro, L., Cuesta-Dominguez, A., Deikus, G., Bernal-Rubio, D., Campisi, L., Fernandez-Sesma, A., Sebra, R., Simon, V., & Mulder, L. C. F. (2018). HIV-1 Infection of Primary CD4(+) T Cells Regulates the Expression of Specific Human Endogenous Retrovirus HERV-K (HML-2) Elements. Journal of Virology, 92(1). DOI
- Lee, H., Pine, P. S., McDaniel, J., Salit, M., & Oliver, B. (2016). External RNA Controls Consortium Beta Version Update. Journal of Genomics, 4, 19-22. DOI
- Bolisetty, M. T., Rajadinakaran, G., & Graveley, B. R. (2015). Determining exon connectivity in complex mRNAs by nanopore sequencing. Genome Biology, 16, 204. DOI
- Frith, M. C., Hamada, M., & Horton, P. (2010). Parameters for accurate genome alignment. BMC Bioinformatics, 11, 80. DOI
- de Jong, L. C., Cree, S., Lattimore, V., Wiggins, G. A. R., Spurdle, A. B., kConFab, I., Miller, A., Kennedy, M. A., & Walker, L. C. (2017). Nanopore sequencing of full-length BRCA1 mRNA transcripts reveals co-occurrence of known exon skipping events. Breast Cancer Research, 19(1), 127. DOI
- Li, H., & Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25(14), 1754-1760. DOI
- Weirather, J. L., de Cesare, M., Wang, Y., Piazza, P., Sebastiano, V., Wang, X. J., Buck, D., & Au, K. F. (2017). Comprehensive comparison of Pacific Biosciences and Oxford Nanopore Technologies and their applications to transcriptome analysis. F1000Research, 6, 100. DOI
- Fu, S., Ma, Y., Yao, H., Xu, Z., Chen, S., Song, J., & Au, K. F. (2018). IDP-denovo: de novo transcriptome assembly and isoform annotation by hybrid sequencing. Bioinformatics, 34(13), 2168-2176. DOI
- Moldovan, N., Szucs, A., Tombacz, D., Balazs, Z., Csabai, Z., Snyder, M., & Boldogkoi, Z. (2018). Multiplatform next-generation sequencing identifies novel RNA molecules and transcript isoforms of the endogenous retrovirus isolated from cultured cells. FEMS Microbiology Letters, 365(5). DOI
- Moldovan, N., Tombacz, D., Szucs, A., Csabai, Z., Snyder, M., & Boldogkoi, Z. (2017). Multi-Platform Sequencing Approach Reveals a Novel Transcriptome Profile in Pseudorabies Virus. Frontiers in Microbiology, 8, 2708. DOI
- Hargreaves, A. D., & Mulley, J. F. (2015). Assessing the utility of the Oxford Nanopore MinION for snake venom gland cDNA sequencing. PeerJ, 3, e1441. DOI
- Marchet, C., Lecompte, L., Silva, C. D., Cruaud, C., Aury, J. M., Nicolas, J., & Peterlongo, P. (2018). De novo clustering of long reads by gene from transcriptomics data. Nucleic Acids Research. DOI
- Kent, W. J. (2002). BLAT--the BLAST-like alignment tool. Genome Research, 12(4), 656-664. DOI
- Oikonomopoulos, S., Wang, Y. C., Djambazian, H., Badescu, D., & Ragoussis, J. (2016). Benchmarking of the Oxford Nanopore MinION sequencing for quantitative and qualitative assessment of cDNA populations. Scientific Reports, 6, 31602. DOI
- Jain, M., Fiddes, I. T., Miga, K. H., Olsen, H. E., Paten, B., & Akeson, M. (2015). Improved data analysis for the MinION nanopore sequencer. Nature Methods, 12(4), 351-356. DOI
- Chaisson, M. J., & Tesler, G. (2012). Mapping single molecule sequencing reads using basic local alignment with successive refinement (BLASR): application and theory. BMC Bioinformatics, 13, 238. DOI
- Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403-410. DOI
- Smith, T. F., & Waterman, M. S. (1981). Identification of common molecular subsequences. Journal of Molecular Biology, 147(1), 195-197
- Sovic, I., Sikic, M., Wilm, A., Fenlon, S. N., Chen, S., & Nagarajan, N. (2016). Fast and sensitive mapping of nanopore sequencing reads with GraphMap. Nature Communications, 7, 11307. DOI
- Wagner, G. P., Kin, K., & Lynch, V. J. (2012). Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory in Biosciences, 131(4), 281-285. DOI