Preparation and Properties of Antitumor Drug Based on Lipid Derivative of Sarcolysin
1Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: burova13@gmail.com
2Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences,
16/10, bld.7 Miklukho-Maklay, Moscow, 117198 Russia
Keywords: sarcolysin; lipid derivative; phospholipid nanoparticles; oncology
DOI: 10.18097/BMCRM00098
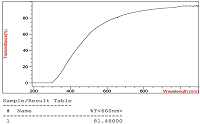
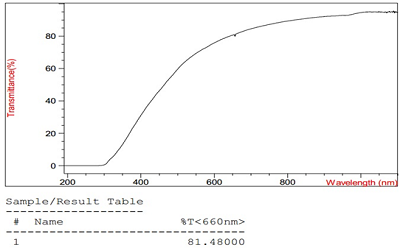
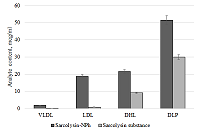
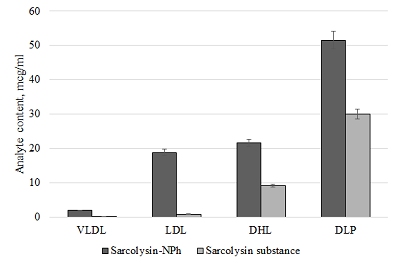
The conditions for the preparation of a drug formulations based on the lipid derivative of sarcolysin embedded in phospholipid nanoparticles have been optimized. The drug is an ultra-thin emulsion with a light transmittance above 80% and a particle size of not more than 50 nm. It should be noted that 99% of the lipid derivative of sarcolysin are incorporated into phospholipid nanoparticles. Preservation of aggregation stability in the aquatic environment was observed for at least 2 days. In vitro experiments have shown that sarcolysin, introduced as a part of phospholipid nanoparticles, is distributed among lipoproteins and protein components of plasma. Moreover, the content of sarcolysin in all fractions involved in the transport of biologically active substances in the body, is significantly higher in case of prodrug administration (lipid derivative of sarcolysin) in the composition of phospholipid nanoparticles than, as compared with administration of a free form (pharmacological substances) to the incubation medium. The transformation of a prodrug into the drug sarcolysin occurs in the blood cells.
|
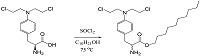
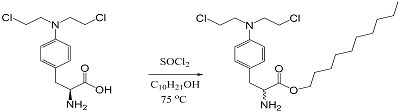
Figure 2.
General reaction scheme for the synthesis of the lipid derivative sarcolysin with decanol.
|

|
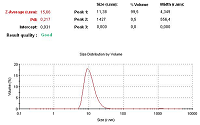
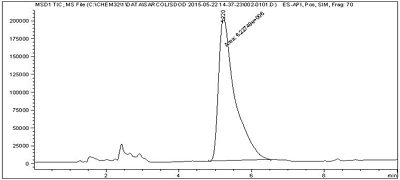
Figure 5.
Chromatogram of an analysis of a drug suspension on the content of a lipid derivative of sarcolysin in it by LC / MS.
|
|
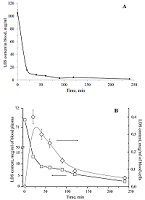
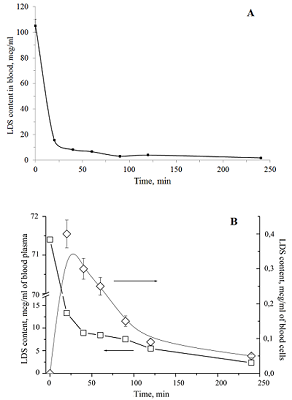
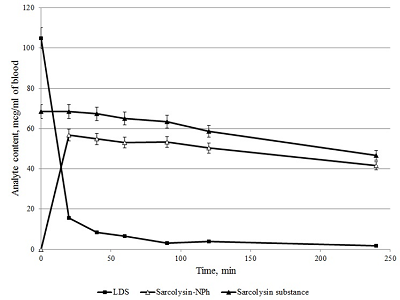
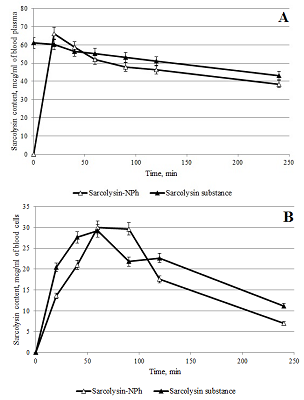
Figure 9.
Changes in the content of sarcolysin in plasma (A) and blood form elements (B) after adding to the whole blood Sarcolysin-NPh (white symbols) and sarcolysin (black symbols).
|
|
CLOSE

|
Table 1.
Characteristics of emulsion samples obtained at various technological parameters.
|
|
CLOSE

|
Table 2.
The change in particle diameter in a unimodal distribution, polydispersity index and light transmission at a wavelength of 660 nm in samples of drug emulsion.
|
FUNDING
The work was financially supported by the Ministry of Education and Science of the Russian Federation, under the State Contract № 14.N08.11.1029 «Preclinical studies of a drug based on a lipid derivative of sarcolysin in a liposomal form for the treatment of myeloma and hemoblastosis».
REFERENCES
- Xiaomei, M., Herbert, Y. (2006) Global Burden of Cancer. Yale J. Biol. Med, 79 (3-4), 85–94.
- Karpunin, V.O., Klenov, G.I., Khoroshkov, B. C. (2008) Russia's first specialized clinical center for proton radiation therapy. Clinical Medicine Almanac, 17 (1), 316-319.
- Thomas, D, Radhakrishnan, P. (2019) Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer, 18 (1), 14. DOI
- Warwi, G. P. (1963) The Mechanism of Action of Alkylating Agents. Cancer Research, 23, 1315-1333.
- Baryshnikov, A.Yu., Zimakova, N.A., Kikot, B.S. (2001) Chemical and pharmaceutical research of a new drug form of sarcolysine. Chemical-pharmaceutical journal, 12, 42-47.
- Zhukova, O.V., Bulgakova, S.A., Khokhlova, T.L. (2012) Some approaches to the creation of pharmacologically active polymer derivatives of drugs with antitumor activity. Pharmateca. Oncology, 18 (251), 14-19.
- Bogomilova, A., Höhn, M., Günther, M., Herrmann, A., Troev, K., Wagner, E., Schreiner, L. (2013) A polyphosphoester conjugate of melphalan as antitumoral agent. Eur. J. Pharm. Sci., 50(3-4), 410-419.
- Baryshnikov, A. Yu. (2012) Nanostructured liposomal systems as a means of delivery of antitumor drugs. Bulletin of the Russian Academy of Medical Sciences, 67 (3), 23-31.
- Panahi, Y., Farshbaf, M., Mohammadhosseini, M., Mirahadi, M., Khalilov, R., Saghfi, S., Akbarzadeh, A. (2017) Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artificial Cells, Nanomedicine, and Biotechnology, 45 (4), 788-799. DOI
- Hrkach, J.., Von Hoff, D., Mukkaram, A. M., Andrianova, E., Auer, J., Campbell, T., De Witt, D., Figa, M., Figueiredo, M., Horhota, A., Low, S., McDonnell, K., Peeke, E., Retnarajan, B., Sabnis, A., Schnipper, E., Song, J.J., Song, Y.H., Summa, J., Tompsett, D., Troiano, G., Van Geen Hoven, T., Wright, J., LoRusso, P., Kantoff, P.W., Bander, N.H., Sweeney, C., Farokhzad, O.C., Langer, R., Zale, S. (2012) Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med., 4, 128-139. DOI
- Kolishetti, N., Dhar, S., Valencia, P.M., Lin, L.Q., Karnik, R., Lippard, S.J., Langer, R., Farokhzad, O.C. (2010) Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. U. S. A., 107, 17939-17944. DOI
- Chang, H.I., Yeh, M.K. (2012) Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomedicine, 7, 49-60. DOI
- Hamaguchi, T.., Kato, K., Yasui, H., Morizane, C., Ikeda, M., Ueno, H., Muro, K., Yamada, Y., Okusaka, T., Shirao, K., Shimada, Y., Nakahama, H., Matsumura, Y. (2007) A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br. J. Cancer, 97, 170-176. DOI
- Egusquiaguirre, S.P., Igartua, M., Hernández, R.M. Pedraz, J.L . (2012) Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin. Transl. Oncol., 14 (2), 83-93. DOI: 10.1007/s12094-012-0766-6
- Svistelnik, A.V., Khanin, A. L. (2014) Liposomal drugs: opportunities and prospects. Medicine in Kuzbass, 2, 7-16.
- Bielawski, K., Bielawska, A. (2008) Small-molecule based delivery systems for alkylating antineoplastic compounds. ChemMedChem, 3(4), 536-542. DOI
- Vodovozova, E.L., Gaenko, G.P., Bobrikova, E.S., Pazynina, G.V., Molotkovsky, Yul.G. (2007) Diglyceride derivative of methotrexate: synthesis and cytotoxic activity in targeted liposomes. Chemical-pharmaceutical journal, 41(6), 10-14. DOI
- Zanette, M., Marcomini, A., Marchiori, E., Samperi, R. (1996) High-performance liquid chromatographic-fluorescence determination of aliphatic alcohol polyethoxylates and polye(thyleneglycol)s in aqueous samples. Journal of Chromatography A., 756 (1–2), 159-174. DOI
- Shironin, A.V., Ipatova, O.M., Medvedeva, N.V., Prozorovskiy, V.N., Tikhonova, E.G., Zakharova, T.S., Sanzhakov, M.A., Torhowskaya, T.I. (2011) Increased bioavailability and anti-inflammatory efficacy of indomethacin when embedded in phospholipid nanoparticles. Biomedical chemistry, 57(6), 671-676. DOI
- Sanzhakov, M.A., Prozorovsky, V.N., Ipatova, O.M., Tikhonova, E.G., Medvedeva, N.V., Torkhovskaya, T.I. (2013) Transport system based on phospholipid nanoparticles for rimfapicin. Biomedical Chemistry, 59 (8), 585-590.
- Guseva, D.A., Khudoklinova, Yu.Yu., Medvedeva, N.V., Baranova, V.S., Zakharova, T.S., Artyushkova, E.B., Torkhovskaya, T.I., Ipatova, O.M. (2015) The effect of incorporation of resveratrol and dihydroquercetin into phospholipid nanoparticles on their bioavailability and specific activity. Biomedical Chemistry, 61 (5), 598-605. 10.18097/PBMC20156105598 ' target='_blank' > DOI
- Medvedeva, N.V., Torkhovskaya, T.I., Kostryukova, L.V., Zakharova, T.S., Kudinov, V.A., Kasatkina, E.O., Prozorovsky, V.N., Ipatova, O.M. (2017) The effect of the incorporation of doxorubicin into phospholipid nanoparticles on tumor accumulation and specific activity. Biomedical Chemistry, 63 (1), 56-61. DOI
- Sanzhakov, M.A., Ipatova, O.M., Torkhovskaya, T.I., Tikhonova, E.G., Medvedeva, N.V., Zakharova, T.S., Prozorovsky, V.N. (2018) Increasing the anti-tuberculosis effect of rifampicin upon incorporation into phospholipid nanoparticles with sodium oleate. Biomedical Chemistry, 64 (6), 505-510. DOI
- Medvedeva, N.V. Prozorovskiy, V.N., Ignatov, D.V., Druzhilovskaya, O.S., Kudinov, V.A., Kasatkina, E.A., Tikhonova, E.G., Ipatova, O.M. (2015) Drugs and transport nanosystems based on plant phospholipids. Biomedical chemistry, 61 (2), 219-230. DOI
- The State Pharmacopoeia of Russian Federation. 12 th ed. (2007) Moscow: Federal State Budgetary Institution «Scientific Centre for Expert Evaluation of Medicinal Products» of the Ministry of Health of the Russian Federation.
- GOST R 56702-2015. (2016) Medicines for medical applications. Nonclinical toxicology and pharmacokinetic studies of safety. Moscow: Standartinform.
- Masquelier, M., Vitols, S., Peterson, C. (1986) Low-density lipoprotein as a carrier of antitumoral drugs: in vivo fate of drug-human low-density lipoprotein complexes in mice. Cancer Res., 46 (8), 3842-3847.
- Dutta, R.C. (2007) Drug carriers in pharmaceutical design: promises and progress. Curr. Pharm. Res., 13 (7), 761-769.
- Torhovskaya, T.I., Ipatova, O.M., Medvedeva, N.V., Ivanov, V.S., Ivanova, L.I. (2010) Blood plasma lipoproteins as carriers of drugs. Effect of phospholipid dosage forms. Bulletin of the Russian Academy of medical Sciences, 5, 42-50.
- Shironin, A.V., Ipatova, O.M., Medvedeva, N.V., Prozorovskiy, V.N., Tikhonova, E.G., Torhovskaya, T.I. (2012) Injectable form of indomethacin in phospholipid nanoparticles: Association with low density lipoproteins and anti-inflammatory action. Efferent and physico-chemical medicine, 1, 21-24.
- Zykova, M.A., Ipatova, O.M., Prozorovskiy, V.N., Medvedeva, N.V. Voskresenskaya, A.A., Zakharova, T.S., Torhowskaya, T.I. (2011) Changes in the distribution of doxorubicin in blood and plasma when it is included in the phospholipid nanocomposition. Biomedical chemistry, 57 (2), 174-179. DOI
- Wasan, K.M., Ramaswamy, M., Cassidy, S.M., Kazemi, M., Strobel, F.W., Thies, R.L. (1997) Physical characteristics and lipoprotein distribution of liposomal nystatin in human plasma. Antimicrob. Agents Chemother, 41 (9), 1871-1875.
- Ahmed, A.E., Hsu, T.F., El-Azhary, R.A., Moawad, H., Costanzi, J. (1982) Macromolecular interactions of [14c-ring]melphalan in blood. Biochem. Pharmacology, 31 (8), 1615-1619.