Inhibition of Caspase-2 Activity in Human Jurkat T-Cell Lymphoma Cells by Splice Switching Oligonucleotide to its pre-mRNA
1Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: zhdanovdd@mail.ru
2Peoples Friendship University of Russia, 6 Miklukho-Maklaya str., Moscow, 117198 Russia
Keywords: caspase-2; alternative splicing; splice switching oligonucleotide; enzymatic activity
DOI:10.18097/BMCRM00108
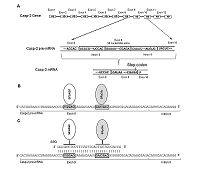
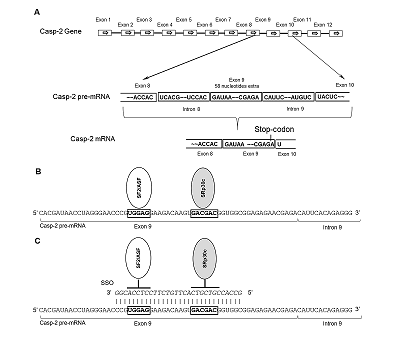
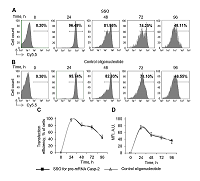
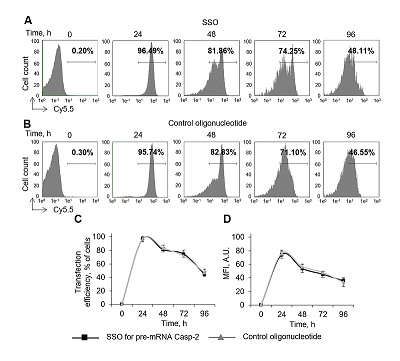
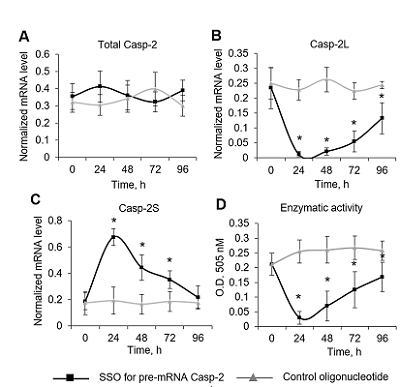
Caspase-2 is a key enzyme thinvolved in induction of apoptosis. The caspase-2 level is regulated by alternative splicing (AS) of its mRNA. The aim of this work was to determine the ability of an oligonucleotide complementary to Casp-2 pre-mRNA to induce AS. This oligonucleotide blocked the binding of splicing-regulating proteins to their sites at the end of exon 9 of Casp-2 pre-mRNA, leading to induction of AS of Casp-2 mRNA. The decrease in expression of full-size active splice-variant (Casp-2L) and the increase the expression of a shortened variant (Casp-2S) was demonstrated in human T-cell lymphoma Jurkat cell line. The expression level of total Casp-2 remained unchanged. Disproportion of splice variants of Casp-2 led to inhibition of enzymatic activity of caspase-2.
|
CLOSE

|
Table 1.
Oligonucleotides used for the transfection of Jurkat cells.
|
|
CLOSE

|
Table 2.
Primers used for real-time RT-PCR.
|
FUNDING
The work was performed in the framework of the Program for Basic Research of the State Academies of Sciences for 2013–2020.
REFERENCES
- Bao, Q., Shi, Y. (2007) Apoptosome: a platform for the activation of initiator caspases. Cell Death and Differentiation, 14(1), 56–65. DOI
- Vakifahmetoglu-Norberg, H., Zhivotovsky, B. (2010) The unpredictable caspase-2: what can it do? Trends in Cell Biology, 20(3), 150–159. DOI
- Wotawa, A., Solier, S., Logette, E., Solary, E., Corcos, L. (2002) Differential influence of etoposide on two caspase-2 mRNA isoforms in leukemic cells. Cancer Letters, 185(2), 181–189. DOI
- Aravind, L., Dixit, V.M., Koonin, E.V. (1999) The domains of death: evolution of the apoptosis machinery. Trends in Biochemical Sciences, 24(2), 47–53. DOI
- Zhivotovsky, B., Orrenius, S. (2005) Caspase-2 function in response to DNA damage. Biochemical and Biophysical Research Communications, 331(3), 859–867. DOI
- Wang, L., Miura, M., Bergeron, L., Zhu, H., Yuan, J. (1994) Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell, 78(5), 739–750. DOI
- Kumar, S., Kinoshita, M., Noda, M. (1997) Characterization of a mammalian cell death gene Nedd2. Leukemia, 11(3), 385–386. DOI
- Lavrik, I.N., Golks, A., Baumann, S., Krammer, P.H. (2006) Caspase-2 is activated at the CD95 death-inducing signaling complex in the course of CD95-induced apoptosis. Blood, 108(2), 559–565. DOI
- Jiang, Z.H., Zhang, W.J., Rao, Y., Wu, J.Y. (1998) Regulation of Ich-1 pre-mRNA alternative splicing and apoptosis by mammalian splicing factors. Proc. Natl. Acad. Sci. USA, 95(16), 9155–9160. DOI
- Côté, J., Dupuis, S., Wu, J.Y. (2001) Polypyrimidine track-binding protein binding downstream of caspase-2 alternative exon 9 represses its inclusion. Journal of Biological Chemistry, 276(11), 8535–8543. DOI
- Havlioglu, N., Wang, J., Fushimi, K., Vibranovski, M.D., Kan, Z., Gish, W., Wu, J.Y. (2007) An intronic signal for alternative splicing in the human genome. PloS one, 2(11), e1246. DOI
- Fushimi, K., Ray, P., Kar, A., Wang, L., Sutherland, L.C., Wu, J.Y. (2008) Up-regulation of the proapoptotic caspase 2 splicing isoform by a candidate tumor suppressor, RBM5. Proc. Natl. Acad. Sci. USA, 105(41), 15708–15713. DOI
- Rocha, C.S.J. (2019) Antisense Oligonucleotides for Splice Modulation: Assessing Splice Switching Efficacy. Methods Mol. Biol. 2036, 73–90. DOI
- Zhdanov, D.D., Gladilina, Y.A., Grishin, D.V., Pokrovsky, V.S., Pokrovskaya, M.V., Aleksandrova, S. S., Sokolov, N. N. (2018) Apoptotic Endonuclease EndoG Induces Alternative Splicing of Telomerase TERT Catalytic Subunit, Caspase-2, DNase I, and BCL-x in Human, Murine, and Rat CD4+T Lymphocytes. Russian Journal of Bioorganic Chemistry, 44(1), 90–103. DOI
- Zhdanov, D.D., Vasina, D.A., Orlova, V.S., Gotovtseva, V.Y., Bibikova, M. V., Pokrovsky, V.S., Sokolov, N.N. (2016) Apoptotic endonuclease EndoG induces alternative splicing of telomerase catalytic subunit hTERT and death of tumor cells. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 10(4), 310–321. DOI
- Zhdanov, D.D., Gladilina, Y.A., Pokrovsky, V.S., Grishin, D.V., Grachev, V.A., Orlova, V.S., Sokolov, N.N. (2019) Endonuclease G modulates the alternative splicing of deoxyribonuclease 1 mRNA in human CD4+ T lymphocytes and prevents the progression of apoptosis. Biochimie, 157, 158–176. DOI
- Baker, B.F., Lot, S.S., Condon, T.P., Cheng-Flournoy, S., Lesnik, E.A., Sasmor, H.M., Bennett, C. F. (1997) 2’-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. Journal of Biological Chemistry, 272(18), 11994–12000. DOI
- Rigo, F., Seth, P.P., Bennett, C.F. (2014) Antisense oligonucleotide-based therapies for diseases caused by pre-mRNA processing defects. Advances in Experimental Medicine and Biology, 825, 303–352. DOI
- Havens, M.A., Hastings, M.L. (2016) Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Research, 44(14), 6549–6563. DOI