The Sturgeon Ovarian Liquid and the Perch Roe Extract are Able to Enhance the Recovery of the Fibroblasts after their Stress-induced Premature Senescence
1Institute of Biomedical Chemistry, 8119121 Pogodinskaya Str., 10 bldg. 8, Moscow, Russia,*e-mail: fireaxe@mail.ru 2ChNIORH LLC, 10-83 Slavy prospect, St. Petersburg, 192239 Russia
Key words: fibroblasts; premature senescence; oxidative stress; fish roe; fish ovarian liquid
DOI: 10.18097/BMCRM00011
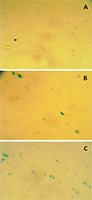
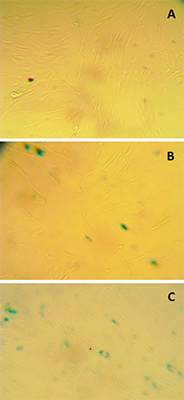
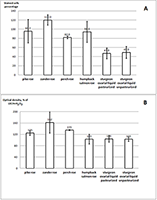
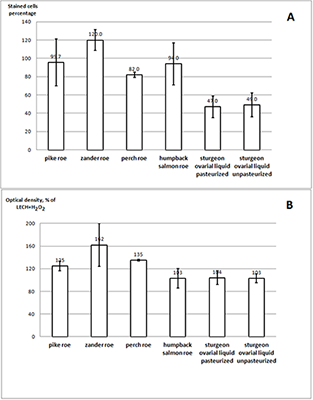
Ovarian liquid and fish roe are valuable sources of biologically active compounds. In order to study mechanisms of aging and also to search for biologically active compounds capable of inhibiting aging, we have modelled premature senescence in human embryonic fibroblasts by incubating of cells with Н2О2. Cell senescence was assessed by staining for β-galactosidase (SA-β-Gal) at pH 6.0; cell proliferation was further evaluated by the optical method. The dried ovarian liquid of the Siberian sturgeon and the extract of the perch roe were able to enhance recovery of the cells after induction of their premature senescence caused by oxidative stress. In contrast to the extract from perch roe and the extracts from fish muscle, dried ovarian fluid gave such an effect without the growth of proliferation.
ACKNOWLEDGEMENTS
This work was performed within the framework of the Program of Fundamental Scientific Research of the State Academies of Sciences of Russia for 2013 – 2020.
REFERENCES
- Campisi, J. (2013). Aging, cellular senescence, and cancer. Annual Review of Physiology, 75, 685–705. DOI
- Senthil, K.K., Gokila, V.M., Mau, J.L., Lin, C.C., Chu, F.H, Wei C.C, Liao, V.H, Wang, S.Y. (2016). A steroid like phytochemical Antcin M is an anti-aging reagent that eliminates hyperglycemia-accelerated premature senescence in dermal fibroblasts by direct activation of Nrf2 and SIRT-1. Oncotarget, 7(39), 62836–62861. DOI
- Dimri, G.P., Lee, X., Basile, G, Acosta, M., Scott, G., Roskelley, C., Medrano, E.E., Linskens, M., Rubelj, I., Pereira-Smith, O., Peacocke, M., Campisi, J. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92(20), 9363–9367.
- Van Deursen, J.M. (2014). The role of senescent cells in ageing. Nature, 509(7501), 439–446. DOI
- Cho, E.J., Okamoto, T., Yokozawa, T. (2008). Therapeutic efficacy of Kangen-karyu against H2O2-induced premature senescence. Journal of Pharmacy and Pharmacology, 60(11), 1537–1544. DOI
- Chen, Q.M., Tu, V.C., Catania, J., Burton, M., Toussaint, O., Dilley, T. (2000). Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide. Journal of Cell Science, 113, 4087–4097.
- Chen, J.H., Stoeber, K., Kingsbury, S., Ozanne, S.E., Williams, G.H, Hales, C.N. (2004). Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. Journal of Biological Chemistry, 279(47), 49439–49446. DOI
- Argyropoulou, A., Aligiannis, N., Trougakos, I.P., Skaltsounis, A.L. (2013). Natural compounds with anti-ageing activity. Natural Product Reports, 30(11), 1412–1437. DOI
- Marotta, F., Polimeni, A., Solimene, U., Lorenzetti, A., Minelli, E., Jain, S., Rastmanesh, R., Sedriep, S., Soresi, V. (2012). Beneficial modulation from a high-purity caviar-derived homogenate on chronological skin aging. Rejuvenation Research, 15(2), 174–177. DOI
- Yoshino, A., Polouliakh, N., Meguro, A., Takeuchi, M., Kawagoe, T., Mizuki, N. (2016). Chum salmon egg extracts induce upregulation of collagen type I and exert antioxidative effects on human dermal fibroblast cultures. Clinical Interventions in Aging, 11, 1159–1168. DOI
- Lahnsteiner, F., Weismann, T, Patzner, R.A. (1995). Composition of the ovarian fluid in 4 salmonid species: Oncorhynchus mykiss, Salmo trutta f lacustris, Saivelinus alpinus and Hucho hucho. Reproduction Nutrition Development, 35, 465–474. DOI
- Nynca, J., Arnold, G.J., Fröhlich, T., Ciereszko, A. (2015). Shotgun proteomics of rainbow trout ovarian fluid. Reproduction, Fertility and Development, 27(3), 504–512. DOI
- Sytova, M.V., Kharenko, E.N. (2007). Ovarian liquid of sturgeon fishes - perspective raw material for receiving of biologically active substances. Rybprom, (3), 41–43.
- Seehuus, S.C., Norberg, K., Gimsa, U., Krekling, T., Amdam, G.V. (2006). Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proceedings of the National Academy of Sciences of the United States of America, 103(4), 962–967. DOI
- Garcia, J., Munro, E.S., Monte, M.M., Fourrier, M.C., Whitelaw, J., Smail, D.A., Ellis, A.E. (2010). Atlantic salmon (Salmo salar L.) serum vitellogenin neutralises infectivity of infectious pancreatic necrosis virus (IPNV). Fish and Shellfish Immunology, 29(2), 293–297. DOI
- Itzhaki, R.F., Gill, D.M. (1964). A micro-biuret method for estimating proteins. Analytical Biochemistry, 9(4), 401–410. DOI
- Wang, Z., Wei, D., Xiao, H. (2013). Methods of cellular senescence induction using oxidative stress. Methods in Molecular Biology, 1048, 135–144. DOI
- Mikhailova, M.V., Belyaeva, N.F., Kozlova, N.I., Zolotarev, K.V., Mikhailov, A.N., Berman, A.E., Archakov, A.I. (2017). Protective action of fish muscle extracts against cellular senescence induced by oxidative stress. Biomeditsinskaya Khimiya, 63(4), 351–355. DOI
- Simboeck, E., Di Croce, L. (2013). p16INK4a in cellular senescence. Aging, 5(8), 590–601. DOI
- Demidenko, Z.N., Zubova, S.G., Bukreeva, E.I., Pospelov, V.A., Pospelova, T.V., Blagosklonny, M.V. (2009). Rapamycin decelerates cellular senescence. Cell Cycle, 8(12), 1888–1895. DOI
- Blagosklonny, M.V. (2012). Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging, 4(3), 159–165. DOI
- Demidenko, Z.N., Blagosklonny, M.V. (2009). At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle, 8(12), 1901–1904. DOI
- Botden, I.P., Oeseburg, H., Durik, M., Leijten, F.P., Van Vark-Van Der Zee, L.C., Musterd-Bhaggoe, U.M., Garrelds, I.M., Seynhaeve, A.L., Langendonk, J.G, Sijbrands, E.J., Danser, A.H., Roks, A.J. (2012). Red wine extract protects against oxidative-stress-induced endothelial senescence. Clinical Science, 123(8), 499–507. DOI
- Yang, Y., Li, S. (2015). Dandelion Extracts Protect Human Skin Fibroblasts from UVB Damage and Cellular Senescence. Oxidative Medicine and Cellular Longevity, 2015, 619560. DOI
- Kim, Y.J., Cha, H.J., Nam, K.H., Yoon, Y., Lee, H., An, S. Kim,Y.J., Cha, H.J., Nam, K.H., Yoon, Y., Lee, H., An, S. (2011). Centella asiatica extracts modulate hydrogen peroxide-induced senescence in human dermal fibroblasts. Experimental Dermatology, 20(12), 998–1003. DOI