Transcriptional Analysis of HeLa Cells - Producers of the Recombinant Peptidoglycan Recognition Protein PGLYRP1 at Different Stages of the Chlamydia Trachomatis Infection Development
Federal Research and Clinical Center of Physical-Chemical Medicine,
1a Malaya Pirogovskaya str., Moscow, 119435 Russia; *e-mail: pbobrovskiy@gmail.com
Keywords:Chlamydia trachomatis; PGLYRP; recombinant proteins; transcriptomics
DOI:10.18097/BMCRM00113
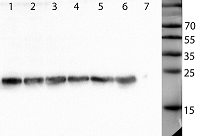
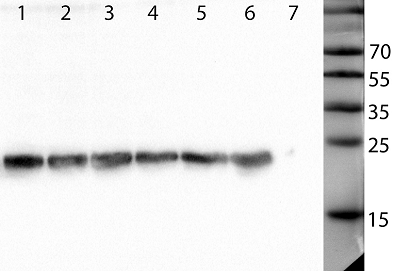
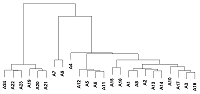
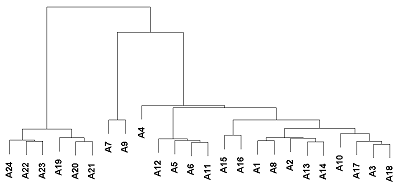
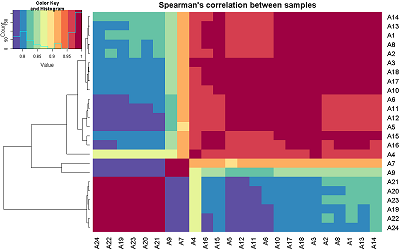
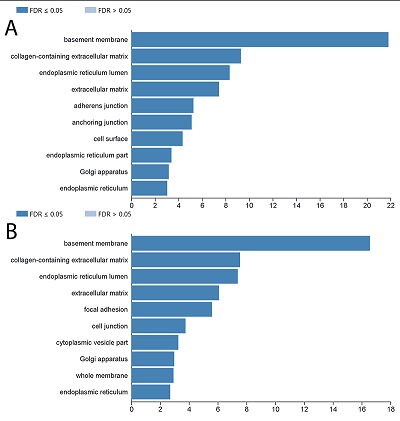
Human peptidoglycan recognition proteins (PGLYRPs) are the components of innate immunity that exhibit antibacterial activity. In this study a cell line secreting recombinant PGLYRP1 into a culture medium was obtained. Transcriptional profiling of cell lines expressing PGLYRP1 was performed at different stages of C. trachomatis infection. Differential gene expression was studied using the whole transcriptome profiling method on the HumanHT-12 v4 Expression BeadChip microchip using the Illumina Direct Hybridization Whole-Gene Expression Assay protocol. Sample clustering followed by bioinformatics analysis revealed about 100 differentially expressed genes in response to infection with C. trachomatis. PGLYRP1- expressing cells infected with C. trachomatis had a similar transcriptional profile as non-infected cells.
|
CLOSE

|
Table 1.
Scheme of an experiment of transcriptome profiling of cell lines expressing PGLYRP1 after infection with C. trachomatis at different stages of infection.
|
|
CLOSE

|
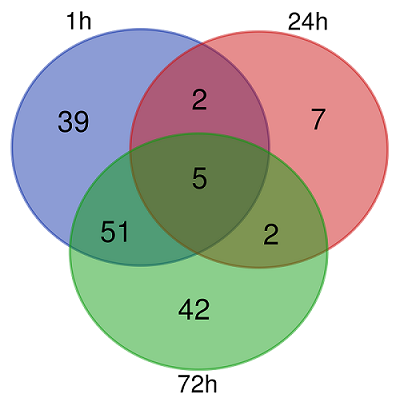
Table 2.
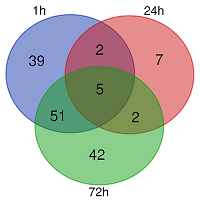
List of common differentially expressed genes in HeLa-PGLYRP1m cell line 1, 24 and 72 hours after infection with C. trachomatis.
|
SUPPLEMENTARY
Supplementary materials are available at http://dx.doi.org/10.18097/BMCRM00113
REFERENCES
- Liu, C., Xu, Z., Gupta, D., & Dziarski, R. (2001). Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. The Journal of Biological Chemistry, 276(37), 34686–34694. DOI
- Kang, D., Liu, G., Lundstrom, A., Gelius, E., & Steiner, H. (1998). A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proceedings of the National Academy of Sciences of the United States of America, 95(17), 10078–10082. DOI
- Royet, J., & Dziarski, R. (2007). Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nature Reviews. Microbiology, 5(4), 264–277. DOI
- Tydell, C. C., Yuan, J., Tran, P., & Selsted, M. E. (2006). Bovine peptidoglycan recognition protein-S: antimicrobial activity, localization, secretion, and binding properties. Journal of Immunology (Baltimore, Md. : 1950), 176(2), 1154–1162. DOI
- Lu, X., Wang, M., Qi, J., Wang, H., Li, X., Gupta, D., & Dziarski, R. (2006). Peptidoglycan recognition proteins are a new class of human bactericidal proteins. The Journal of Biological Chemistry, 281(9), 5895–5907. DOI
- Dziarski, R., Kashyap, D. R., & Gupta, D. (2012). Mammalian peptidoglycan recognition proteins kill bacteria by activating two-component systems and modulate microbiome and inflammation. Microbial Drug Resistance (Larchmont, N.Y.), 18(3), 280–285. DOI
- Dziarski, R., & Gupta, D. (2018). How innate immunity proteins kill bacteria and why they are not prone to resistance. Current Genetics, 64(1), 125–129. DOI
- Moulder, J. W. (1991). Interaction of chlamydiae and host cells in vitro. Microbiological Reviews, 55(1), 143–190.
- Hearn, S. A., & McNabb, G. L. (1991). Immunoelectron microscopic localization of chlamydial lipopolysaccharide (LPS) in McCoy cells inoculated with Chlamydia trachomatis. The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society, 39(8), 1067–1075. DOI
- Liechti, G. W., Kuru, E., Hall, E., Kalinda, A., Brun, Y. V, VanNieuwenhze, M., & Maurelli, A. T. (2014). A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature, 506(7489), 507–510. DOI
- Bobrovsky, P., Manuvera, V., Polina, N., Podgorny, O., Prusakov, K., Govorun, V., & Lazarev, V. (2016). Recombinant human peptidoglycan recognition proteins reveal antichlamydial activity. Infection and Immunity, 84(7). DOI
- Scidmore, M. A. (2005). Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Current Protocols in Microbiology, Chapter 11, Unit 11A.1. DOI
- Sambrook, J., Fritsch, E. F., & Maniatis, T. , Molecular Cloning: A Laboratory Manual. 1989, Cold Spring Harbor Laboratory Press. 1-3.
- R Development Core Team. (2009). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved from http://www.r-project.org
- Haynes, W. (2013). Benjamini--Hochberg Method. In W. Dubitzky, O. Wolkenhauer, K.-H. Cho, & H. Yokota (Eds.), Encyclopedia of Systems Biology (p. 78). DOI
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. Retrieved from https://ggplot2.tidyverse.org
- Neuwirth, E. (2014). RColorBrewer: ColorBrewer Palettes
- Bioinformatics & Evolutionary Genomics. (2019). Retrieved November 27, 2019, from http://bioinformatics.psb.ugent.be/webtools/Venn/
- Heinzen, R. A., & Hackstadt, T. (1997). The Chlamydia trachomatis parasitophorous vacuolar membrane is not passively permeable to low-molecular-weight compounds. Infection and Immunity, 65(3), 1088–1094.
- Stephens, A. J., Aubuchon, M., & Schust, D. J. (2011). Antichlamydial antibodies, human fertility, and pregnancy wastage. Infectious Diseases in Obstetrics and Gynecology, 2011, 525182. DOI
- Scidmore, M. A. (2011). Recent advances in Chlamydia subversion of host cytoskeletal and membrane trafficking pathways. Microbes and Infection, 13(6), 527–535. DOI
- Grieshaber, S. S., Grieshaber, N. A., Miller, N., & Hackstadt, T. (2006). Chlamydia trachomatis causes centrosomal defects resulting in chromosomal segregation abnormalities. Traffic (Copenhagen, Denmark), 7(8), 940–949. DOI