The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Effect of the Cycle Size and Spacer Structure in Tacrine and its Cyclopentyl Homologue Conjugates with 5-(4-trifluoromethyl-phenylamino)-1,2,4-thiadiazole on the Spectrum of their Biological Activity
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia,*e-mail: kovalevanv@ipac.ac.ru
Key words: atacrine; 1,2,4-thiadiazoles; acethylcholinesterase; butyrylcholinesterase; antioxidants; Alzheimer’s disease
DOI: 10.18097/BMCRM00027
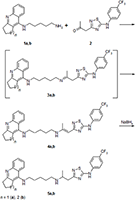
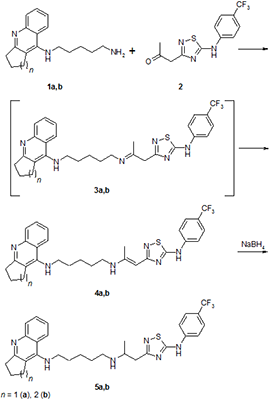
The conjugates of tacrine and its cyclopentyl analogue with 5- (4-trifluoromethyl-phenylamino) -1,2,4-thiadiazole, combined with two different spacers, pentylaminopropane and pentylaminopropene, were synthesized. Their esterase profile, the ability to displace propidium from the peripheral anionic site (PAS) of acetylcholinesterase (AChE) and antioxidant activity in the ABTS test were investigated. The compounds obtained effectively inhibit cholinesterases with a predominant effect on butyrylcholinesterase, displace propidium from the PAS of Electrophorus electricus AChE (EeAChE) and exhibit a high radical-scavenging capacity. It is shown that, depending on the spacer structure, particulary, the presence of a propenamine or propanamine fragment, the spectrum of biological activity of the conjugates changes.
|
CLOSE

|
Table 1.
Inhibitor activity and selectivity of conjugates 4a (n=1), 4b (n=2), 5a (n=1), 5b (n=2) toward AChE, BChE and CaE.
|
|
CLOSE

|
Table 2.
The ability of conjugates 4a,b and 5а,b to displace propidium from the peripheral anionic site of EeAChE and scavenge free radicals in the ABTS test.
|
ACKNOWLEDGEMENTS
This work was performed within the framework of the RFBR grant #17-03-00984-а (synthesis, structure determination, as well as study of the esterase profile of the compounds obtained and molecular modeling ). Displacement of propidium iodide from the PAS of EeAChE and ABTS scavenging activity of conjugates were studied in the framework of the Russian State assignment № 0090-2017- 0019. The equipment of the Center for the collective use of IPAС RAS was used. Molecular modeling was carried out using the equipment of the Center for the collective use of super high-performance computing resources of the Lomonosov Moscow State University.
REFERENCES
- Hamulakova, S., Poprac, P., Jomova, K., Brezova, V., Lauro, P., Drostinova, L., Jun, D., Sepsova, V., Hrabinova, M., Soukup, O., Kristian, P., Gazova, Z., Bednarikova, Z., Kuca, K., & Valko, M. (2016). Targeting copper(II)-induced oxidative stress and the acetylcholinesterase system in Alzheimer's disease using multifunctional tacrine-coumarin hybrid molecules. Journal of Inorganic Biochemistry, 161, 52-62. DOI
- Zhang, C., Du, Q.-Y., Chen, L.-D., Wu, W.-H., Liao, S.-Y., Yu, L.-H., & Liang, X.-T. (2016). Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as multi-targeted compounds against Alzheimer's disease. European Journal of Medicinal Chemistry, 116, 200-209. DOI
- Bachurin, S. O., Bovina, E. V., & Ustyugov, A. A. (2017). Drugs in Clinical Trials for Alzheimer's Disease: The Major Trends. Medicinal Research Reviews, 37(5), 1186-1225. DOI
- Rosini, M., Simoni, E., Minarini, A., & Melchiorre, C. (2014). Multi-target design strategies in the context of Alzheimer's disease: acetylcholinesterase inhibition and NMDA receptor antagonism as the driving forces. Neurochemical Research, 39(10), 1914-1923. DOI
- Minarini, A., Milelli, A., Simoni, E., Rosini, M., Bolognesi, M., Marchetti, C., & Tumiatti, V. (2013). Multifunctional Tacrine Derivatives in Alzheimer's Disease. Current Topics in Medicinal Chemistry, 13(15), 1771-1786. DOI
- Guzior, N., Wi.eckowska, A., Panek, D., & Malawska, B. (2014). Recent Development of Multifunctional Agents as Potential Drug Candidates for the Treatment of Alzheimer’s Disease. Current Medicinal Chemistry, 22(3), 373-404. DOI
- Tonelli, M., Catto, M., Tasso, B., Novelli, F., Canu, C., Iusco, G., Pisani, L., Stradis, A. D., Denora, N., Sparatore, A., Boido, V., Carotti, A., & Sparatore, F. (2015). Multitarget Therapeutic Leads for Alzheimer's Disease: Quinolizidinyl Derivatives of Bi- and Tricyclic Systems as Dual Inhibitors of Cholinesterases and beta-Amyloid (Abeta) Aggregation. ChemMedChem, 10(6), 1040-1053. DOI
- Li, Y., Geng, J., Liu, Y., Yu, S., & Zhao, G. (2013). Thiadiazole-a promising structure in medicinal chemistry. ChemMedChem, 8(1), 27-41. DOI
- Castro, A., Castano, T., Encinas, A., Porcal, W., & Gil, C. (2006). Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles. Bioorganic & Medicinal Chemistry, 14(5), 1644-1652. DOI
- Martinez, A., Fernandez, E., Castro, A., Conde, S., Rodriguez-Franco, I., Baños, J.-E., & Badia, A. (2000). N-Benzylpiperidine derivatives of 1,2,4-thiadiazolidinone as new acetylcholinesterase inhibitors. European Journal of Medicinal Chemistry, 35(10), 913-922. DOI
- Porcal, W., Hernandez, P., Gonzalez, M., Ferreira, A., Olea-Azar, C., Cerecetto, H., & Castro, A. (2008). Heteroarylnitrones as drugs for neurodegenerative diseases: synthesis, neuroprotective properties, and free radical scavenger properties. Journal of Medicinal Chemistry, 51(19), 6150-6159. DOI
- Makhaeva, G. F., Proshin, A. N., Boltneva, N. P., Rudakova, E. V., Kovaleva, N. V., Serebryakova, O. G., Serkov, I. V., & Bachurin, S. O. (2016). 1,2,4-Thiadiazoles as promising multifunctional agents for treatment of neurodegenerative diseases. Russian Chemical Bulletin, 65(6), 1586-1591. DOI
- Makhaeva, G. F., Grigoriev, V. V., Proshin, A. N., Kovaleva, N. V., Rudakova, E. V., Boltneva, N. P., Serkov, I. V., & Bachurin, S. O. (2017). Novel Conjugates of Tacrine with 1,2,4,-Thiadiazole as Highly Effective Cholinesterase Inhibitors, Blockers of NMDA Receptors, and Antioxidants Doklady Biochemistry and Biophysics, 477, 405–409. DOI
- Ellman, G. L., Courtney, K. D., Andres, V., Jr., & Feather-Stone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88-95.
- Sterri, S. H., Johnsen, B. A., & Fonnum, F. (1985). A radiochemical assay method for carboxylesterase, and comparison of enzyme activity towards the substrates methyl [1-14C] butyrate and 4-nitrophenyl butyrate. Biochemical Pharmacology, 34(15), 2779-2785.
- Taylor, P., Lwebuga-Mukasa, J., Lappi, S., & Rademacher, J. (1974). Propidium—a Fluorescence Probe for a Peripheral Anionic Site on Acetylcholinesterase. Molecular Pharmacology, 10(4), 703-708.
- Taylor, P., & Lappi, S. (1975). Interaction of fluorescence probes with acetylcholinesterase. Site and specificity of propidium binding. Biochemistry, 14(9), 1989-1997. DOI
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9-10), 1231-1237. DOI
- Szymanski, P., Laznickova, A., Laznicek, M., Bajda, M., Malawska, B., Markowicz, M., & Mikiciuk-Olasik, E. (2012). 2,3-dihydro-1H-cyclopenta[b]quinoline derivatives as acetylcholinesterase inhibitors-synthesis, radiolabeling and biodistribution. International Journal of Molecular Sciences, 13(8), 10067-10090. DOI
- Serkov, I. V., Proshin, A. N., Petrova, L. N., & Bachurin, S. O. (2010). Novel 1,2,4-thiadiazoles with an NO-producing fragment. Doklady Chemistry, 435(2), 311-313. DOI
- Mesulam, M.-M., Guillozet, A., Shaw, P., Levey, A., Duysen, E.G., & Lockridge, O. (2002) . Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience, 110 (4), 627-639 DOI
- Nordberg, A., Ballard, C., Bullock, R., Darreh-Shori, T., & Somogyi, M. (2013). A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer's disease. The Primary Care Companion for CNS Disorders, 15(2). DOI
- Lane, R. M., Potkin, S. G., & Enz, A. (2006). Targeting acetylcholinesterase and butyrylcholinesterase in dementia. The International Journal of Neuropsychopharmacology, 9(1), 101-124. DOI
- Greig, N. H., Utsuki, T., Ingram, D. K., Wang, Y., Pepeu, G., Scali, C., Yu, Q. S., Mamczarz, J., Holloway, H. W., Giordano, T., Chen, D., Furukawa, K., Sambamurti, K., Brossi, A., & Lahiri, D. K. (2005). Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer b-amyloid peptide in rodent. Proceedings of the National Academy of Sciences, 102(47), 17213-17218. DOI
- Inestrosa, N. C., Dinamarca, M. C., & Alvarez, A. (2008). Amyloid-cholinesterase interactions. Implications for Alzheimer's disease. The FEBS journal, 275(4), 625-632. DOI
- Grigoriev, V. V., Makhaeva, G. F., Proshin, A. N., Kovaleva, N. V., Rudakova, E. V., Boltneva, N. P., Gabrel´yan, A. V., Lednev, B. V., & Bachurin, S. O. (2017). 1,2,4-Thiadiazole derivatives as effective NMDA receptor blockers with anticholinesterase activity and antioxidant properties. Russian Chemical Bulletin, 66(7), 1308-1313. DOI
- Proshin, A. N., Serkov, I. V., Petrova, L. N., & Bachurin, S. O. (2014). 5-Amino-3-(2-aminopropyl)-1,2,4-thiadiazoles as the basis of hybrid multifunctional compounds. Russian Chemical Bulletin, 63(5), 1148-1152. DOI