The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Properties of Calcium-Activated Chloride Currents in Rat Purkinje Cerebellum Neurons
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: vzam@yandex.ru
Key words: patch-clamp method; Purkinje cerebellum cells; calcium-activated chloride current; tetraethylammonium; 4-aminopyridin; nifuminic acid
DOI: 10.18097/BMCRM00034
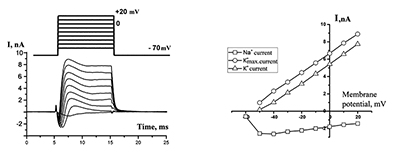
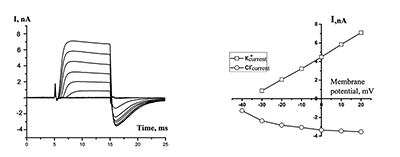
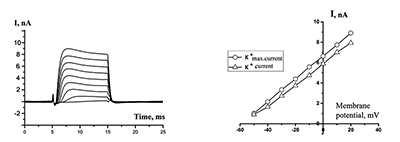
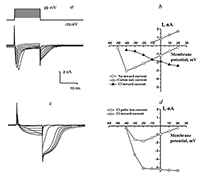
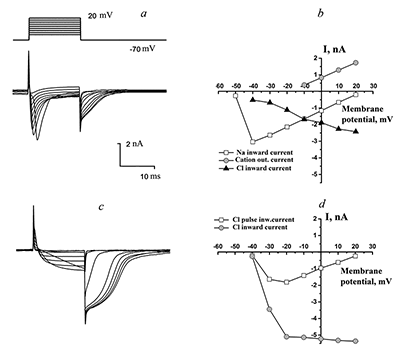
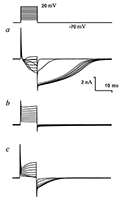
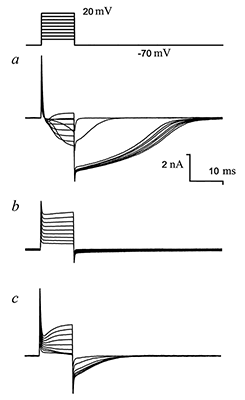
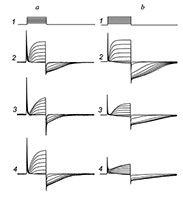
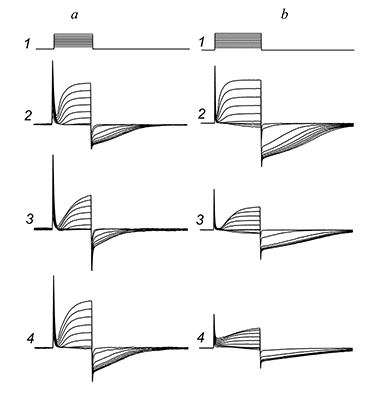
The presence of calcium-activated chloride current was shown using on freshly isolated rat Purkinje cerebellum neiurones and the pacth-clamp method in the whole-cell configuration. Chloride currents appeared in sodium-free external solution and reversibly disappeared in chloride-free or calcium-free external solution. Replacing of K+ ions (120 mM) to Cs+ ions in micropipette (120 mM) show the chloride currents even with 140 mM Na+ in external solution. This current was blocked to 80–100% by nifluminic acid (25–100 μM). It was found out that well known blockers of potassium channels tetraethylammonium (TEA) and 4-aminopyridine (4-AP) also effectively blocked chloride channels. The IC50 values for TEA and 4-AP were 130 μM, and 110 μM respectively. The action of TEA was reversible, while 4-AP at concentration 100 μM and above irreversibly blocked chloride channels.
|
CLOSE

|
Table 1.
Ionic composition of solutions.
|
ACKNOWLEDGEMENTS
The equipment of the Centre of collective usage of IPAS RAS was used in the work (Agreement No. 14.621.21.0008,
work identifier RFMEFI62114X0008).
REFERENCES
- Pedemonte, N. Galietta, L. J. V. (2014). Structure and Function of Tmem16 Proteins (Anoctamins). Physiological Reviews, 94(2): p. 419-459. DOI
- Huang, F., Rock, J. R., Harfe, B. D., Cheng, T., Huang, X. Z., Jan, Y. N., Jan, L. Y. (2009). Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proceedings of the National Academy of Sciences of the United States of America, 106(50): p. 21413-21418. DOI
- Kaneda, M., Nakamura, H., Akaike, N. (1988). Mechanical and Enzymatic Isolation of Mammalian Cns Neurons. Neuroscience Research, 5(4): p. 299-315. DOI
- Hamill, O. P., Marty, A., Neher, E., Sakmann, B., Sigworth, F. J. (1981). Improved Patch-Clamp Techniques for High-Resolution Current Recording from Cells and Cell-Free Membrane Patches. Pflugers Archiv-European Journal of Physiology, 391(2): p. 85-100. DOI
- Ferrera, L., Caputo, A., Galietta, L. J. V. (2010). TMEM16A Protein: A New Identity for Ca2+-Dependent Cl- Channels. Physiology, 25(6): p. 357-363. DOI
- Arroyo, J. P., Kahle, K. T., Gamba, G. (2013). The SLC12 family of electroneutral cation-coupled chloride cotransporters. Molecular Aspects of Medicine, 34(2-3): p. 288-298. DOI
- Kaila, K., Price, T. J., Payne, J. A., Puskarjov, M., Voipio, J. (2014). Cation-chloride cotransporters in neuronal development, plasticity and disease. Nature Reviews Neuroscience, 15(10): p. 637-654. DOI
- Huang, W. C., Xiao, S. H., Huang, F., Harfe, B. D., Jan, Y. N., Jan, L. Y. (2012). Calcium-Activated Chloride Channels (CaCCs) Regulate Action Potential and Synaptic Response in Hippocampal Neurons. Neuron, 74(1): p. 179-192. DOI
- Sanchez, D. Y. Blatz, A. L. (1994). Block of Neuronal Fast Chloride Channels by Internal Tetraethylammonium Ions. Journal of General Physiology, 104(1): p. 173-190. DOI