The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Crown-Containing Phthalocyanines are Potential Sensibilizers for Photodynamic Therapy.
Synthesis, Properties and Role of Non-Covalent Interactions
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: mager1988@gmail.com
2Institute of Problems of Chemical Physics of Russian Academy of Sciences, 1 Acad. Semenova av., Chernogolovka, Moscow region, 142432 Russia
3Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, 31 Leninsky av., Moscow, 119071 Russia
Key words: phthalocyanines; photodynamic therapy; microheterogeneous environments; spectroscopy
DOI: 10.18097/BMCRM00042
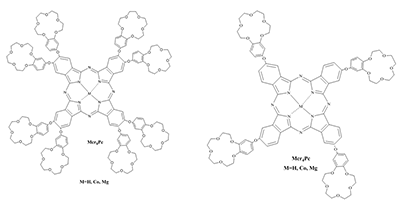
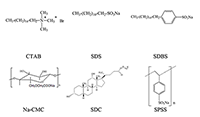
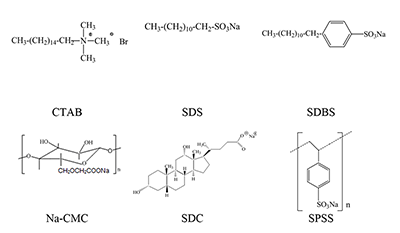
Phthalocyanines (Pc) and their supramolecular aggregates are widely used in molecular electronics, chemical sensors, and catalysis, as well as in biology and medicine, including photodynamic therapy (PDT). One of the possibilities of preventing Pc aggregation in an aqueous medium is using surfactants: with their molecules are self-organized various supramolecular complexes. This results in formation of the required microheterogeneous Pc environment compatible with the biological medium. The monomolecular state of Pc in an aqueous medium is especially important for their use as sensitizers in fluorescence diagnostics and PDT. We have summarized here the results of investigations of distinctive features of the supramolecular aggregation of octa-[(4′-benzo-15-crown-5)oxy]phthalocyaninates (Mcr8Pc) and tetra-[(4′-benzo-15-crown-5)oxy]phthalocyaninates (Mcr4Pc) in electrolytic solutions and solutions of synthetic cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), sodium dodecylbenzenesulfonate (SDBS). Biocompatible surfactants such as carboxymethylcellulose sodium salt (Na-CMC) and sodium deoxycholate (SDC) were also studied. Using the electron absorption spectra it has been shown that formation of Mcr8Pc monomers in micellar solutions of SDC is affected by both increased surfactant concentration and by changes in the ionic strength of solution after sodium chloride is added. The effect of the chemical structure of the biocompatible anionic surfactant on monomerization of crown_containing phthalocyanines has been identified; this fact opens new possibilities for using this family of compounds for fluorescent diagnosis and PDT.
|
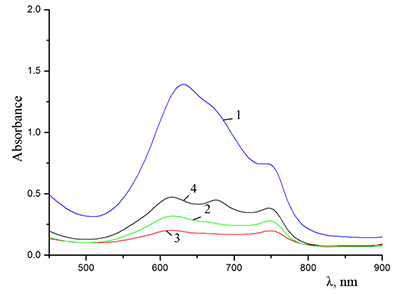
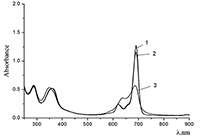
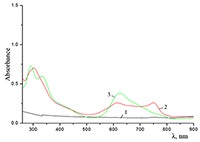
Figure 5.
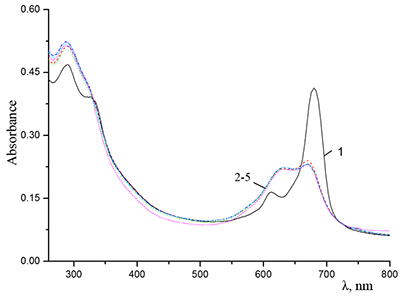
Absorption spectra of (a) (1) CTAB, (2) Cocr8Pc + CTAB, and (3) H2cr8Pc + CTAB at Cocr8Pc, H2cr8Pc, and CTAB concentrations of
1.25•10–5, 9•10–6, and 8.6•10–3 M, respectively. |
|
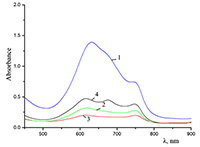
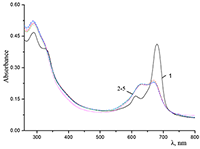
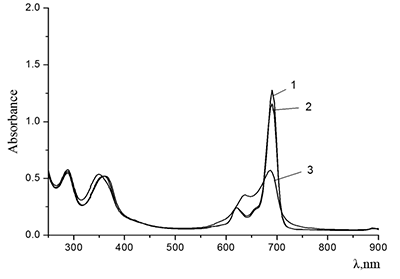
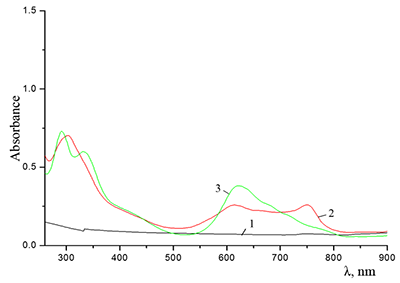
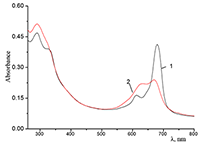
Figure 6.
Cocr8Pc in aqueous solutions: (1) 8.6•10–3 M CTAB; (2) 1•10–3 M CTAB +4.2•10–3 M KCl; and (3) 5.5•10–2 M KCl at Cocr8Pc concentration, M: (1, 2) 1.25•10–5 and (3) 1.3•10–5.
|
|
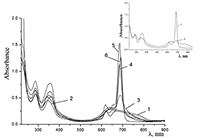
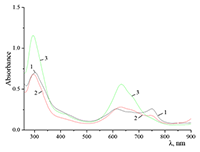
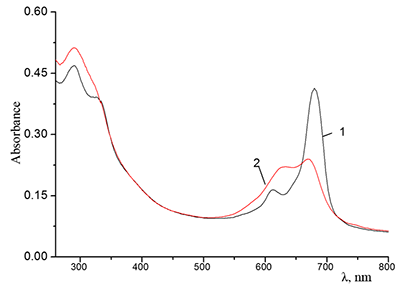
Figure 7.
Absorption spectra of 0.67•10–5 M Cocr4Pc in (1) CH2Cl2 and (2) mixed C2H5OH + CH2Cl2 solvent at a 1:1 volume ratio of the components.
|
|
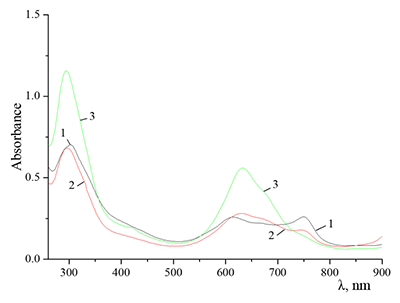
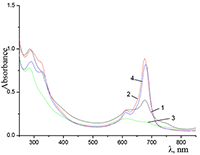
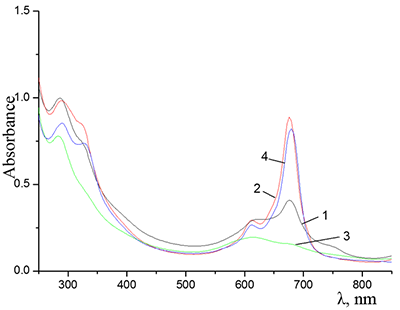
Figure 9.
Absorption spectra of Cocr4Pc in (1) 0.01 M and (2) 0.085 M aqueous SDS, (3) aqueous 0.0086 M CTAB, and (4) dichloromethane.
|
|
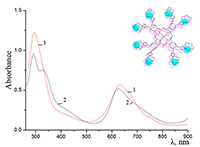
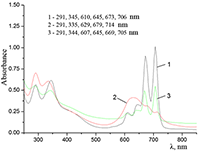
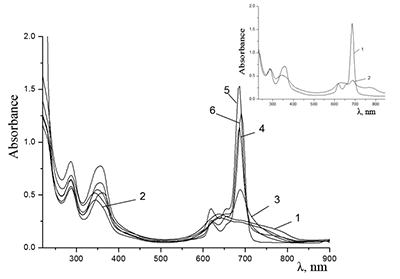
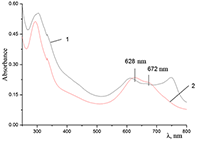
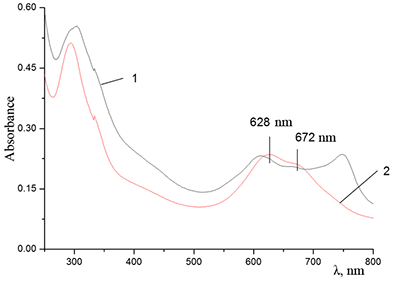
Figure 11.
Absorption spectra of (a) aqueous solutions: (1) Cocr8Pc + Na-CMC and Cocr8Pc + Na-CMC + KCl at a KCl concentration of 0.42 M.
|
|
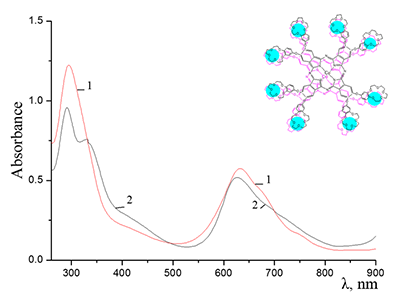
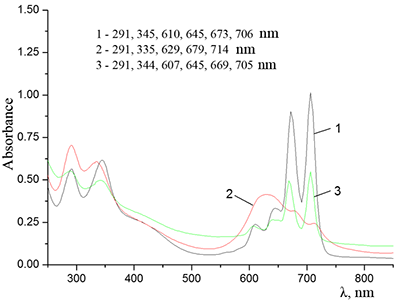
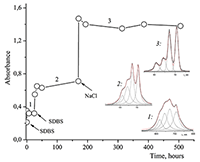
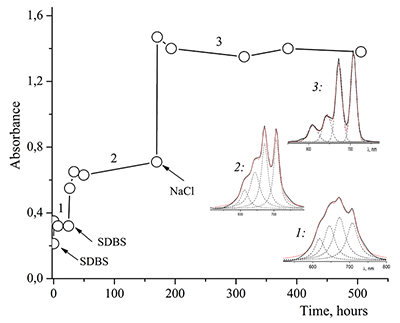
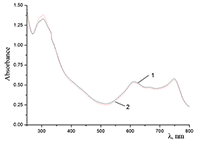
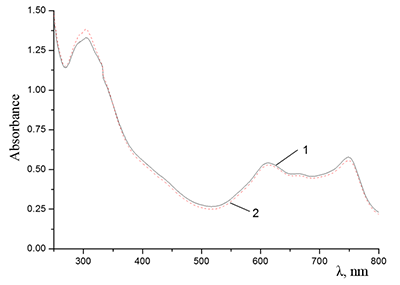
Figure 12.
Absorption spectra of Cocr8Pc + Na-CMC and (2) Cocr8Pc + Na-CMC + NaCl. The spectra were taken with respect to air, solvent is H2O, cuvette optical path is 0.2 cm.
|
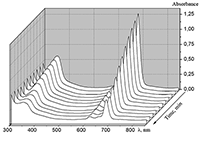
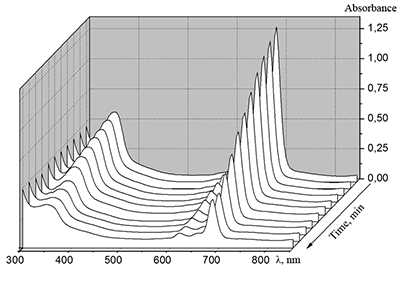
|
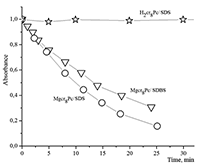
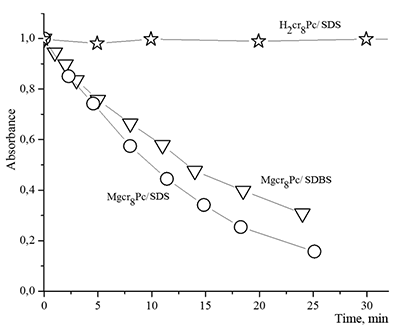
Figure 16.
Changes in absorption spectra of the Mgcr8Pc/SDBS micellar solution in time under illumination.
|
ACKNOWLEDGEMENTS
The work was financially supported by Federal Agency of Scientific Organizations (grants No. 0090-2017-0024 and No. 0081-2014-0015), Russian Academy of Sciences (program No. 34) and partially supported by the RFBR (grants No. 18-03-00743).
REFERENCES
- Rechtman, E., Ciulla, T. A., Criswell, M. H., Pollack A., Harris, A. (2002). An update on photodynamic therapy in age-related macular degeneration. Expert Opin. Pharmacother, 3(7), 931-938. DOI
- Lukyanets, E. A. (1999). Phthalocyanines as Photosensitizers in the Photodynamic Therapy of Cancer. J. Porphyrins Phthalocyanines, 3(6), 424-433. DOI
- Wang, S., Gao, R., Zhou, F., Selke, M. (2004). Nanomaterials and singlet oxygen photosensitizers: potential applications in photodynamic therapy. J. Mater. Chem., 14(4), 487-493. DOI
- Zhang, X.F., Xi, Q., Zhao, J. (2010). Fluorescent and triplet state photoactive J-type phthalocyanine nano assemblies: controlled formation and photosensitizing properties. J. Mater. Chem., 20(32), 6726-6733. DOI
- Lang, K., Kubat, P., Mosinger, J., Wagnerova, D.M. (1998). Photochemical consequences of porphyrin and phthalocyanine aggregation on nucleoprotein histone. J. Photochem. Photobiol. A: Chem. 119(1), 47-52. DOI
- Tsivadze, A. Yu (2004). Supramolecular metal complex systems based on crown-substituted tetrapyrroles. Russ Chem. Rev., 73 (1), 6-25 DOI
- Logacheva, N. M., Baulin, V. E., Tsivadze, A. Yu., Basova, T. V., Sheludyakova, L. A. (2008). Synthesis and spectroscopic study of the d-metal complexes with new octa(benzo-15-crown-5)substituted phthalocyanine. Izvestiya Akademii Nauk. Seriya Khimicheskaya, (7), 1439–1447.
- Hamuryudan, E. (2006). Synthesis and solution properties of phthalocyanines substituted with four crown ethers. Dyes and Pigments. 68 (2–3), 151-157. DOI
- Gordon, A.J., Ford, R.A. (1972). A Handbook of practical data, technique, references. N.Y., London, Sydney, Toronto: Wiley & Sons
- Gol’dshleger, N. F. , Kalashnikova, I. P. Baulin, V. E. , Tsivadze, A. Yu. (2011). Octa- and tetra-(benzo-15-crown-5)phthalocyanines in surfactant-containing solutions. Protection of Metals and Physical Chemistry of Surfaces. 47(4), 471–477. DOI
- Gol’dshleger N.F., Lobach, A.S., Gak, V.Yu., Kalashnikova, I.P. Baulin, V.E., Tsivadze, A.Yu. (2014) Magnesium Octa[(4'-Benzo-15-crown_5)oxy]phthalocyaninate in Water Micellar Solutions of Sodium Deoxycholate. Protection of Metals and Physical Chemistry of Surfaces. 50 (5), 496–505. DOI
- Ovsyannikova, E. V., Goldshleger N. F., Kurochkina N.M., Baulin, ., V. E. Tsivadze, A. Yu. and Alpatova N. M. (2010). Behavior of Octa(benzo-15-crown-5)substituted Metal Phthalocyanines in Polar Media and their Immobilization on Solid Supports. Macroheterocycles, 3 (2-3), 125-133. DOI
- Rosen, M. J.( 2004). Surfactants and Interfacial Phenomena. 3rd ed. Wiley_Int., 444. DOI:10.1002/0471670561.
- Goldshleger, N.F., Chernyak, A.V., Kalashnikova, I.P., Baulin, V.E., Tsivadze (2012 ) A.Yu Magnesium Octa(benzo-15-crown-5)phthalocyaninate in the sodium dodecyl sulfate solutions: A study using electron and 1H NMR spectroscopy. Russian Journal of General Chemistry. 82(5), 927-935. DOI
- Lastovoy A. P., Avramenko G.V. (2013). Spectral-Luminescent Properties of the Substituted Tetraazachlorins Solubilized in Solutions of Nonionic Surfactants. Macroheterocycles.6 (2), 137-143. DOI
- Slota, R., Dyrda, G. (2003). UV Photostability of Metal Phthalocyanines in Organic Solvents // Inorg. Chem., 42 (18), 5743-5750. DOI
- Lapkina, L.A., Gorbunova, Y.G., Gil, D.O., Ivanov, V.K., Konstantinov, N.Yu., Tsivadze A.Yu. (2013). Synthesis, spectral properties, cation-induced dimerization and photochemical stability of tetra-(15-crown-5)-phthalocyaninato indium(III). J. Porphyrins Phthalocyanines, 17 (6-7), 564-572. DOI
- Komissarov, A. N., Makarov, D. A., Yuzhakova, O. A., Savvina, L. P. , Kuznetsova, N. A., Kaliya, O. L., Lukyanets, E. А., Negrimovsky V. M. (2012). Synthesis and Some Properties of Phosphonomethyl Substituted Phthalocyanines. Macroheterocycles, 5 (2) 169-174. DOI