The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
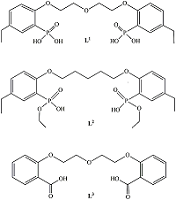
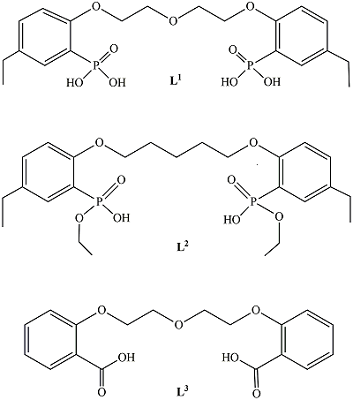
1,5-bis[2-(dioxyphosphoryl)-4-ethylphenoxy]-3-oxapentane and its Analogs as Promising Organic Ligands for Copper(II) Binding
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia,*e-mail: mager1988@gmail.com
2Frumkin Institute of Physical Chemistry and Electrochemistry, RAS, 31/4 Leninskii av., Moscow, 119071 Russia
3Mari State University, 1 Lenin Square, Republic of Mary El, Yoshkar-Ola, 424000 Russia
Key words: diphosphonic acids; dissociation constants; copper(II) complexes; stability constants; potentiometry; spectrophotometry
DOI: 10.18097/BMCRM00043
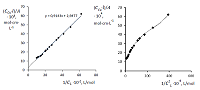
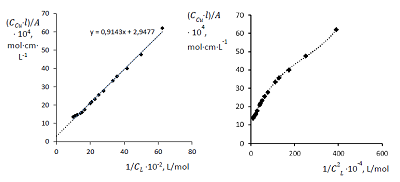
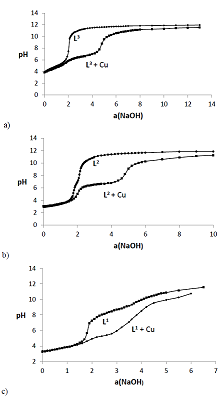
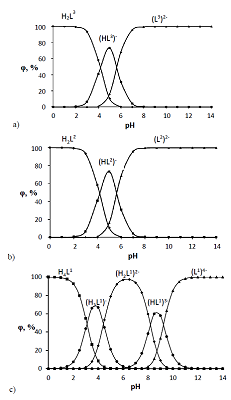
The dissociation and complexation ability toward Cu(II) of acidic type phosphoryl-containing podands – tetrabasic 1,5-bis[2-(dioxyphosphoryl)-4-ethylphenoxy]-3-oxapentane (L1), dibasic – 1,5-bis[2-(ethoxyhydroxyphosphoryl)-4-ethylphenoxy]-pentane (L2) and also of their carboxylic analogue dibasic 1,5-bis[2-(oxycarbonylphenoxy)]-3-oxapentane (L3) were investigated by spectrophotometric, conductometric and potentiometric methods in water in the presence 5% of dimethyl formamide. Spectrophotometric and conductometric titration data provided evidence for formation of 1 : 1 (M : L) complexes. The dissociation constants were determined and species distribution diagrams for studied acids were obtained by potentiometric method. These data are of interest for the design of binary extragents and medicinal drugs based on the studied ligands. The stability constants of Cu(II) 1: 1 (M : L) complexes were estimated. Analysis of the titration curves suggests that deprotonation forms of the studied ligands react with Cu2+. Substitution of carboxylic groups in acyclic polyesters by phosphonic results in increased stability of the copper(II) complexes
|
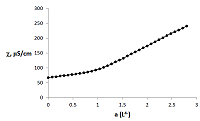
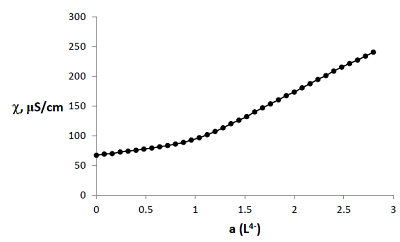
Figure 3.
Curve of conductometric titration of copper(II) solution, a – number of equivalents of added neutralized ligand L4-.
|
|
CLOSE

|
Table 1.
Dissosiation constants of studied acids (µ = 0.1, KCl).
|
|
CLOSE

|
Table 2.
Dissosiation constants of studied acids (µ = 0.1, KCl).
|
ACKNOWLEDGEMENTS
The work was performed on the government assignments no. 0090-2017-0024 and 0081-2014-0015. A part of the work was supported by the Russian Foundation for Basic Research (project no. 18-33-00685) and the Presidium of Russian Academy of Sciences – Russia (program no. 34).
REFERENCES
- Wehbe, M., Leung, A. W. Y., Abrams, M. J., Orvig, C., & Bally, M. B. (2017). A perspective – can copper complexes be developed as a novel class of therapeutics? Dalton Transactions, 46(33), 10758-10773. DOI
- Wadas, T. J., Wong, E. H., Weisman, G. R., & Anderson, C. J. (2010). Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chemical Reviews, 110(5), 2858-2902. DOI
- Cutler, C. S., Hennkens, H. M., Sisay, N., Huclier-Markai, S., & Jurisson, S. S. (2013). Radiometals for combined imaging and therapy. Chemical Reviews, 113(2), 858-883. DOI
- Liu, S. (2008). Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Advanced Drug Delivery Reviews, 60(12), 1347-1370. DOI
- Zulkefeli, M., Suzuki, A., Shiro, M., Hisamatsu, Y., Kimura, E., & Aoki, S. (2011). Selective hydrolysis of phosphate monoester by a supramolecular phosphatase formed by the self-assembly of a bis(Zn2+-cyclen) complex, cyanuric acid, and copper in an aqueous solution (cyclen = 1,4,7,10-tetraazacyclododecane). Inorganic Chemistry, 50(20), 10113-10123. DOI
- Kimura, E. (2012). Evolution of macrocyclic polyamines from molecular science to supramolecular science. Bulletin of Japan Society of Coordination Chemistry, 59, 26-47. DOI
- Meierhofer, T., Rosnizeck, I. C., Graf, T., Reiss, K., Konig, B., Kalbitzer, H. R., & Spoerner, M. (2011). Journal of the American Chemical Society, 133(7), 2048-2051. DOI
- Pierre, V. C., Harris, S. M., & Pailloux, S. L. (2018). Comparing strategies in the design of responsive contrast agents for magnetic resonance imaging: a case study with copper and zinc. Accounts in Chemical Research, 51(2), 342-351. DOI
- Harris, S. M., Srivastava, K., League, A. B., Ziebarth, K. E., & Pierre, V. C. (2018). Achieving selectivity for copper over zinc with luminescent terbium probes bearing phenanthridine antennas. Dalton Transactions, 47(7), 2202-2213. DOI
- Puranik, R., Bao, S., Bonin, A. M., Kaur, R., Weder, J. E., Casbolt, L., Hambley, T. W., Lay, P. A., Barter, P. J., & Rye, K.-A. (2016). A novel class of copper(II)- and zinc(II)-bound non-steroidal anti-inflammatory drugs that inhibits acute inflammation in vivo. Cell & Bioscience, 6:9(1), 1-7. DOI
- Hubin, T. J., Amoyaw, P. N.-A., Roewe, K. D., Simpson, N. C., Maples, R. D., Carder Freeman, T. N., Cain, A. N., Le, J. G., Archibald, S. J., Khan, S. I., Tekwani, B. L., Khan, M. O. F. (2014). Synthesis and antimalarial activity of metal complexes of cross-bridged tetraazamacrocyclic ligands. Bioorganic & Medicinal Chemistry, 22(13), 3239-3244. DOI
- Ibragimov, A. B., Ashurov, Z. M., & Tashpulatov, Z. Z. (2017). Synthesis, structure, and fungicidal activity of mono- and binuclear mixed-ligand copper complex with p-nitrobenzoic acid and monoethanolamine. Russian Journal of Coordination Chemistry (Engl. Transl.), 43(6), 380-388. DOI
- Popova, E. V., Domnina, N. S., Vlasov, P. S., & Tyuterev, S. L. (2015). Fungistatic activity of chitosan complexes with 3-d metals. Uspekhi meditsinskoy mikologii (Russ.), 14(14), 360-363.
- Chikisheva, G. E., Medvedev Yu., A., Sapozhnikov, Yu. E., & Kolbin, A. M. (2013). Comparative antifungal activity of some derivatives of a methyl ether 2-benzimidazolylcarbamine acid concerning phytopathogenic and antropozoopathogenic mushrooms. Bashkirskiy khimicheskiy zhurnal (Russ.), 20(3), 108-111.
- Corbridge, D. E. C. (2013). Phosphorus: Chemistry, Biochemistry and Technology. New York: CRC Press.
- Hill, C., in Ion Exchange and Solvent Extraction: A Series of Advances, Vol. 19, Ed. Moyer, B. A. (2010). Boca Raton: CRC Press. p. 119.
- Nash, K. L., Barrans, R. E., Chiarizia, R., Dietz, M. L., Jensen, M. P., Rickert, P. G., Moyer, B. A., Bonnesen, P. V., Bryan, J. C., & Sachleben, R. A. (2000). Fundamental investigations of separations science for radioactive materials. Solvent Extraction and Ion Exchange, 18(4), 605-631. DOI
- Otu, E. O., & Chiarizia, R. (2001). Temperature effects in the extraction of metal ions by dialkyl-substituted diphosphonic acids. I. The Am(III) case. Solvent Extraction and Ion Exchange, 19(5), 885-904. DOI
- Otu, E. O., & Chiarizia, R. (2001). Thermodynamics of the extraction of metal ions by dialkyl-substituted diphosphonic acids. II. The U(VI) and Sr(II) Case. Solvent Extraction and Ion Exchange, 19(6), 1017-1036. DOI
- Nishihama, S., Witty, R. P., Martin, L. R., & Nash, K. L. (2013). Thermodynamic features of benzene-1,2-diphosphonic acid complexes with several metal ions. Solvent Extraction and Ion Exchange, 31(4), 370-383. DOI
- Zalupski, P. R., McAlister, D. R., Stepinski, D. C., & Herlinger, A. W. (2003). Metal extraction by silyl?substituted diphosphonic acids. III. Ester group substituent effects on phosphoryl oxygen basicity. Solvent Extraction and Ion Exchange, 21(3), 331-345. DOI
- Ali, M. B., Ahmed, A. Ya. B. H., Attou, M., Elias, A., & Didi, M. A. (2012). Solvent Extraction and Ion Exchange, 30(5), 469-479. DOI
- Fu, Q., Yang, L., & Wang, Q. (2007). On-line preconcentration with a novel alkyl phosphinic acid extraction resin coupled with inductively coupled plasma mass spectrometry for determination of trace rare earth elements in seawater. Talanta, 72(4), 1248-1254. DOI
- Lumetta, G. J., Sinkov, S. I., Krause, J. A., & Sweet, L. E. (2016). Neodymium(III) Complexes of dialkylphosphoric and dialkylphosphonic acids relevant to liquid–liquid extraction systems. Inorganic Chemistry, 55(4), 1633-1641. DOI 10.1021/acs.inorgchem.5b02524
- Batchu, N. K., Hoogerstraete, T. V., Banerjee, D., & Binnemans, K. (2017). Separation of rare-earth ions from ethylene glycol (+LiCl) solutions by non-aqueous solvent extraction with Cyanex 923. RSC Advances, 7(72), 45351-45362. DOI 10.1039/C7RA09144C
- Shatrava, I. O., Ovchynnikov, V. A., Gubina, K. E., Sliva, T. Yu., Severinovskaya, O. V., Grebenyuk, A. G., Shishkina, S. V., & Amirkhanov, V. M. (2018). Coordination mode of the N-[bis(diethylamino)phosphoryl]benzenesulfonamide ligand in Lu(III) and Ag(I) complexes. Mass spectra, thermal properties and DFT calculations. Polyhedron, 139, 98-106. DOI
- Ignat’eva, T. I., Baulin, V. E., Tsvetkov, E. N., & Raevskij, O. A. (1990). Phosphorus-containing podands. Acidity and complex-forming properties of podands with dioxyphosphinylphenyl terminal groups. Zhurnal Obshchei Khimii (Russ.), 1990, 60(7), 1503-1506.
- Safiulina, A. M., Matveeva, A. G. , Ivanets, D. V., Kudryavtsev, E. M., Grigor?ev, M. S., Baulin, V. E., & Tsivadze, A. Yu. (2015). Phosphoryl-containing acidic podands as extractants for recovery of f-elements. 1. Synthesis and comparison of podands different in polyether chain length and structure. Russian Chemical Bulletin (Engl. Transl.), 64(1), 161-168. DOI
- Safiulina, A. M., Matveeva, A. G. , Ivanets, D. V., Kudryavtsev, E. M., Baulin, V. E., & Tsivadze, A. Yu. (2015). Phosphoryl-containing acidic podands as extractants for recovery of f-elements. 2. Synthesis and comparison of podands different in terminal group structure. Russian Chemical Bulletin (Engl. Transl.), 64(1), 169-171. DOI
- Turanov, A. N., Karandashev, V. K., Baulin, V. E., & Tsivadze, A. Yu. (2014). Extraction of REEs(III), U(VI), and Th(IV) with acidic phosphoryl-containing podands from nitric acid solutions. Radiochemistry (Engl. Transl.), 56(1), 22-26. DOI
- Baulin, V. E., Kovalenko, O. V., Turanov, A. N., Karandashev, V. K., Usolkin, A. N., Yakovlev, N. G., Voroshilov, Yu. A., & Tsivadze, A. Yu. (2015). Acidic phosphoryl podands as components of impregnation-type sorbents for 99Mo recovery from nitric acid solutions. Radiochemistry (Engl. Transl.), 57(1), 61-68. DOI
- Baulin, V. E., Kalashnikova, I. P., Kovalenko, O. V., Baulin, D. V., Usolkin, A. N., & Tsivadze, A. Yu. (2016). Acidic phosphoryl podands as components of extraction chromatography material for selective extraction of promethium-147. Protection of Metals and Physical Chemistry of Surfaces, 52(6), 996-1004. DOI
- Safiulina, A. M., Ivanets, D. V., & Kudryavtsev, E. M. (2018). Zhurnal Neorganicheskoi Khimii, Paper Code: 020/18. DOI
- Polle, E. G. (1974). Ultraviolet spectroscopic determination of the stabilities of weak organic complexes. Russian Chemical Reviews (Engl. Transl.), 43(8), 631-643. DOI
- Bulatov, M. I., & Kalinkin, I. P. (1986). Practical guide on photometric methods of analysis. 5th edition, revised. Leningrad: Khimiya (Russ.).
- Dyatlova, N. M., Temkina, V. Ya., & Kolpakova, I. D. (1970). Complexones. Moscow: Khimiya (Russ.).
- Belova, V. V., Egorova, N. S., Voshkin, A. A., & Khol’kin, A. I. (2015). Extraction of rare earth metals, uranium, and thorium from nitrate solutions by binary extractants. Theoretical Foundations of Chemical Engineering (Engl. Transl.), 49(4), 545-549. DOI
- Baulin, V. E., Kiskin, M. A., Ivanova, I. S., Pyatova, E. N., Baulin, D. V., & Tsivadze, A. Yu. (2012). Synthesis, vibrational spectra, and crystal and molecular structure of copper 1,5-bis[2-(dihydroxyphosphinyl)phenoxy]-3-oxapentane dihydrate [Cu(H2L2)(H2O)2]. Russian Journal of Inorganic Chemistry (Engl. Transl.), 57(5), 671-675. DOI