The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Biological Activity of Alantolactones in Experiments on Cells
Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: klochkov@ipac.ac.ru
Key words: alantolactones; cytotoxicity; cancer cell lines
DOI: 10.18097/BMCRM00047
The results of in vitro studies demonstrate cytotoxic activity alantolactone and isoalantolactone, presented in the elecampane plant (Inula helenium) of the natural sesquiterpene lactones. These compounds show toxicity against human cancer cell lines: MS, A549, HCT116, MCF7, RD and K562. The involvement of the p53 signaling pathway in the death of tumor cells, as well as the contribution of reactive oxygen species (ROS) formation during cell death, was studied.
|
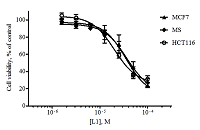
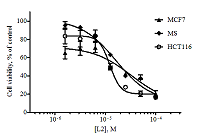
Figure 2.
Dose-dependent curves for alantolactone L1 when exposed to the tumor cell lines obtained as a result of the MTT test (48 hours exposure, n = 3, error bars mean ± SD of the value).
|
|
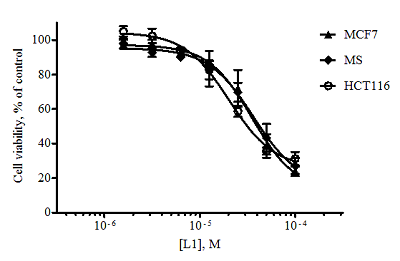
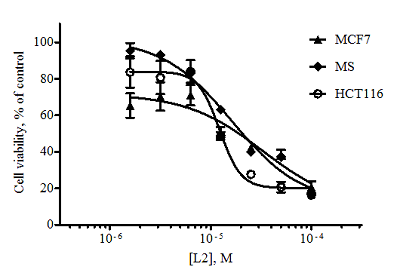
Figure 3.
Dose-dependent curves for isoalantolactone L2 when exposed to the tumor cell lines obtained as a result of the MTT test (48 hours exposure, n = 3, error bars mean ± SD of the value).
|
|
CLOSE

|
Table 1.
Cytotoxicity of allantolactones (MTT test).
|
|
CLOSE

|
Table 2.
Fractal descriptors (Dtot, Dval, Dvdw, Dunb and standard errors Δ) of amino acids (β-sheet).
|
|
CLOSE

|
Table 3.
Survival of MCF7, MS and HCT116 cells under the action of lactone L1 and L2 (100 μM) in the presence and in the presence of N-acetylcysteine (NAC) evaluated by the MTT test (48 hours exposure, n = 3)
|
ACKNOWLEDGEMENTS
The equipment of Сentre of collective usage was used when carrying out study. The work is carried out within the framework of the Government task 0090-2017-0018.
REFERENCES
- Zhang, S., Won, Y.K., Ong, C.N., Shen, H.M. (2005). Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Current Medicinal Chemistry. Anti-Cancer Agents. 5(3), 239–249. DOI
- Marshall, J.A., Cohen, N. (1964). The structure of alantolactone. The Journal of Organic Chemistry. 29(12), 3727–3729. DOI
- Rasul, M., Khan, M., Ali, J., Li, X., Li. A. (2013). Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Scientific World Journal. 5. – ID 248532. DOI
- Klochkov, S.G., Afanas’eva, S.V., Pushin, A.N. (2006). Acidic isomerization of alantolactone derivatives. Chemistry of Natural Compounds, 42(4), 400-406. DOI
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 65(1-2), 55–63. DOI
- Rampersad, S.N. (2012). Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 12(9), 12347–12360, DOI
- Amaral, J.D., Xavier, J.M., Steer, C.J., Rodrigues C.M. (2010). The role of p53 in apoptosis. Discovery Medicine. 45(9), 145–152.
- Cui, L. Bu, W., Song, J., Feng, L., Xu, T., Liu, D., Ding, W., Wang, J., Li, C., Ma, B., Luo, Y., Jiang, Z., Wang, C., Chen, J., Hou, J., Yan, H., Yang, L., Jia, X. (2018) Apoptosis induction by alantolactone in breast cancer MDA-MB-231 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. Archives of Pharmacal Research. 41(3), 299–313. DOI
- Rasul, A., Di, J., Millimouno, F.M., Malhi, M., Tsuji, I., Ali, M., Li, J., Li, X. (2013). Reactive oxygen species mediate isoalantolactone-induced apoptosis in human prostate cancer cells. Molecules. 18(8), 9382–9396. DOI
- Neganova, M.E., Afanas’eva, S.V., Klochkov, S.G., Shevtsova, E.F. (2012). Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bulletin of Experimental Biology and Medicine. 152(6), 720–722. DOI