The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Protective Effects of the Natural Sesquiterpene Lactones Against Damage Induced by Glutamate
and Peroxide in SK-N-MC Cells
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia;*e-mail: dubrovskaya@ipac.ac.ru 2Branch of Talrose Institute of Energetic Problems of Chemical Physics Russian Academy of Sciences, 1-10 Acad. Semenov prosp., Chernogolovka, Moscow region, 142432 Russia
Key words: sesquiterpene lactones; neuroblastoma; peroxide; glutamate; resazurin
DOI: 10.18097/BMCRM00042
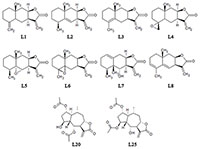
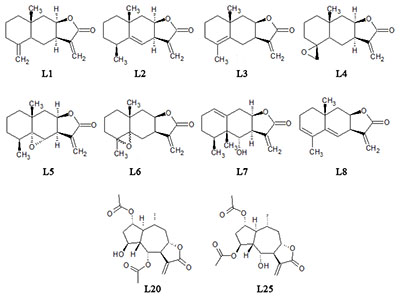
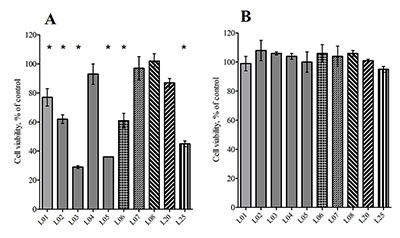
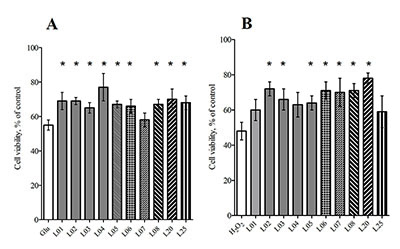
In the present study we evaluated the effect of natural sesquiterpene lactones of plants of the genus Inula on the cells of neuroblastoma SK-N-MC under conditions of toxic stress induced by H2O2 and glutamate. Lactones increased cell survival under these conditions and the leader compounds were identified in this series. A putative mechanism for the protective action of lactones on cells under condition toxic stress has been proposed.
ACKNOWLEDGEMENTS
The equipment of the Center for Collective Use of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences was used. The work was carried out within the framework of the State Proposal 0090-2017-0018.
REFERENCES
- Coyle, J.T. & Puttfarcken, P. (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993, 262, 689-695. DOI
- Cacabelos, R., Takeda, M. & Winblad, B. (1999) The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer's disease. International Journal of Geriatric Psychiatry. 1999, 14, 3–47. DOI
- Lai, T.W., Zhang, S. & Wang, Y.T. (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Progress in Neurobiology. 2014, 115, 157-188. DOI
- Zhao, Y.M., Zhang, M.L., Shi, Q.W. & Kiyota, H. (2006) Chemical constituents of plants from the genus Inula. Chemistry & Biodiversity. 2006, 3(4), 371-384. DOI
- Seo, J.Y., Lim, S.S., Kim, J., Lee, K.W. & Kim, J.S. (2017) Alantolactone and isoalantolactone prevent amyloid β25–35-induced toxicity in mouse cortical neurons and scopolamine-induced cognitive impairment in mice. Phytotherapy Research. 2017, 31(5), 801-811. DOI
- Tambewagh, U.U., Kandhare, A.D., Honmore, V.S., Kadam, P.P., Khedkar, V.M., Bodhankar, S.L. & Rojatkar, S.R. (2017) Anti-inflammatory and antioxidant potential of Guaianolide isolated from Cyathocline purpurea: Role of COX-2 inhibition. International Immunopharmacology. 2017, 52, 110-118. DOI
- Neganova, M.E., Afanas’eva, S.V., Klochkov, S.G. & Shevtsova, E.F. (2012) Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bulletin of Experimental Biology and Medicine. 2012, 152(6), 720-722. DOI
- Klochkov, S.G., Pukhov, S.A., Afanas'eva, S.V., Anikina, L.V. & Ermatova, A.B. (2015) Amination products of Inula britannica lactones and their antitumor activity. Chemistry of Natural Compounds. 2015, 51(3), 435-443. DOI
- Klochkov, S.G., Afanas’eva, S.V. & Pushin, A.N. (2006) Acidic isomerization of alantolactone derivatives. Chemistry of Natural Compounds. 2006, 42(4), 400-406. DOI
- Rampersad, S.N. (2012) Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors, 2012, 12(9), 12347-12360. DOI
- Seo, J.Y., Lim, S.S., Kim, J.R., Lim, J.S., Ha, Y.R., Lee, I.A., Kim, E.J., Park, J.H. & Kim, J.S. (2008) Nrf2-mediated induction of detoxifying enzymes by alantolactone present in Inula helenium. Phytotherapy Research. 2008, 22(11), 1500-1505. DOI
- Chaturvedi, R.K. & Flint Beal, M. (2013) Mitochondrial diseases of the brain. Free Radical Biology and Medicine. 2013, 63, 1–29. DOI
- Zhang, M.J., An, C.R., Gao, Y.Q., Leak, R.K., Chen, J. & Zhang, F. (2013) Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Progress in Neurobiology. 2013, 100, P. 30–47. DOI
- Surh, Y.J. & Na, H.-K. (2008) NF-kB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes & Nutrition. 2008, 2(4), 313-317. DOI