Signal Enhancement of SPR Biosensor Detection by Using of Gold Nanoparticles for Human beta-2-Microglobulin as a Model Protein Biomarker
Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: pavel79@inbox.ru
Key words: surface plasmon resonance (SPR); optical biosensor; signal enhancement; gold nanoparticles; protein-protein interactions
DOI:10.18097/BMCRM00053
The highly sensitive method of surface plasmon resonance (SPR) detection of low concentrations of target proteins based on the biosensor signal enhancement by using gold nanoparticles (similar to «sandwich» assay type) is described. The commercial protein preparations of beta-2-microglobulin (B2M) as a model biomarker and polyclonal (Pab) and monoclonal antibodies (Mab) to B2M were used. It has been shown that this universal and reproducible method can be applied for SPR analysis of other protein biomarkers by analogy with the biomarker protein B2M. The present work is also focused on the experimental protocol description. The protocols of gold nanoparticles (GNP) synthesis, obtaining the conjugates of Pab/GNP and measuring their concentration, the protocol of Mab covalent immobilization on the optical chip CM5 of a biosensor and also SPR registration of molecular interactions Mab-biomarker and in the «sandwich» assay type Mab-biomarker-Pab or Mab-biomarker-Pab/GNP are considered in detail.
|
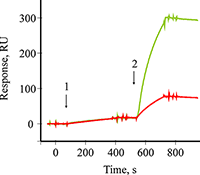
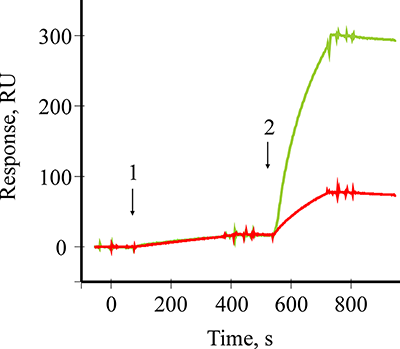
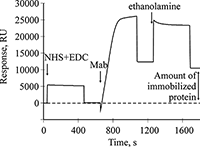
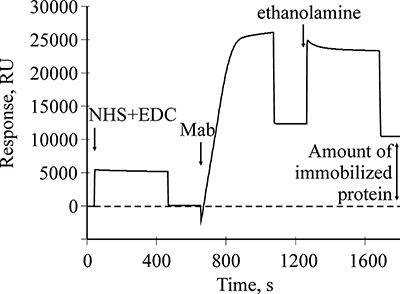
Figure 1.
A sensogramm of covalent amino coupling immobilization of Mab to B2M onto the optical chip CM5.
|
|
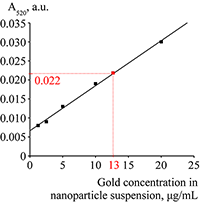
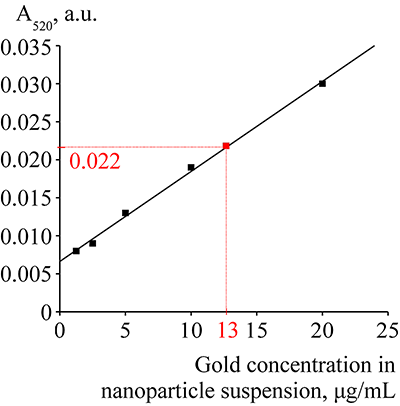
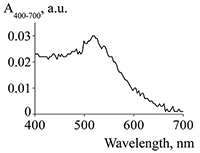
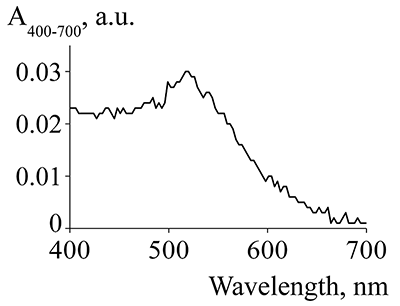
Figure 2.
The absorption spectrum of suspension of gold nanoparticles at a concentration of 20 mkg/mL.
|
|
CLOSE

|
Table 1.
Immobilization protocol for Mab to B2M. Executed commands were derived from the queue window Biacore 3000 Control Software.
|
|
CLOSE

|
Table 2.
Protocol of SPR registration of protein – protein interactions. Executed commands were derived from the queue window Biacore 3000 Control Software.
|
ACKNOWLEDGEMENTS
This work was performed within the framework of the Fundamental Research Program of State Academies of Sciences for 2013-2020. SPR measurements were performed in the “Human Proteome” Core Facility of the Institute of Biomedical Chemistry (Russia, Moscow) which is supported by Ministry of Education and Science of the Russian Federation (unique project ID RFMEFI62117X0017).
REFERENCES
- Homola, J., Vaisocherová, H., Dostálek, J. & Piliarik M. (2005) Multi-analyte surface plasmon resonance biosensing. Methods, 37(1), 26-36. DOI
- Hong, B. & Kang, K.A. (2006) Biocompatible, nanogold-particle fluorescence enhancer for fluorophore mediated, optical immunosensor. Biosensors and Bioelectronics, 21(7), 1333-1338. DOI
- Liu, X., Sun, Y., Song, D., Zhang, Q., Tian, Y., Bi, S., & Zhang, H. (2004) Sensitivity-enhancement of wavelength-modulation surface plasmon resonance biosensor for human complement factor 4. Analytical Biochemistry, 333(1), 99-104. DOI
- Wei, J., Mu, Y., Song, D., Fang, X,, Liu, X,, Bu, L,, Zhang, H., Zhang, G,, Ding, J., Wan,g W., Jin, Q. & Luo, G. (2003) A novel sandwich immunosensing method for measuring cardiac troponin I in sera. Analytical Biochemistry, 321, 209–216. DOI
- Gnedenko, O.V., Mezentsev, Y.V., Molnar, A.A., Lisitsa, A.V., Ivanov, A.S. & Archakov, A.I. (2013) Highly sensitive detection of human cardiac myoglobin using a reverse sandwich immunoassay with a gold nanoparticle-enhanced surface plasmon resonance biosensor. Analytica Chimica Acta, 759, 105-9. DOI
- Dutra, R.F. & Kubota, L.T. (2007) An SPR immunosensor for human cardiac troponin T using specific binding avidin to biotin at carboxymethyldextran-modified goldchip. Clinica Chimica Acta, 376, 114–120. DOI
- Teramura, Y. & Iwata, H. (2007) Label-free immunosensing for alpha-fetoprotein in human plasma using surface plasmon resonance. Analytical Biochemistry, 365(2), 201–207. DOI
- Besselink, G.A., Kooyman, R.P., van Os, P.J., Engbers, G.H. & Schasfoort, R.B. (2004) Signal amplification on planar and gel-type sensor surfaces in surface plasmon resonance-based detection of prostate-specific antigen. Analytical Biochemistry, 333(10), 165–173. DOI
- Kim, M.G., Shin, Y.B. & Jung, J.M. (2005) Enhanced sensitivity of surface plasmon resonance (SPR) immunoassays using a peroxidase-catalyzed precipitation reaction and its application to a protein microarray. Journal of Immunological Methods, 297(1-2), 125-132. DOI
- Wang, M., Wang, L., Wang, G., Ji, X., Bai, Y., Li, T., Gong, S. & Li, J. (2004) Application of impedance spectroscopy for monitoring colloid Au-enhanced antibody immobilization and antibody–antigen reactions. Biosensensors and Bioelectronics, 19(6), 575-582. DOI
- Biacore Sensor Surface Handbook GE Healthcare BR-1005-71 Edition AB 05/2008. P. 39-41.