|
Salivary Cytokine Levels in Lung Cancer with Distant and Regional Metastases
1KhimServis LLC, 4/2 Lugovaya Str., Skolkovo Innovation Center, Moscow, 143026, Russia, *e-mail: ludab2005@mail.ru
2Omsk State Medical University, 12 Lenina Str., Omsk, 644099, Russia
3Strasbourg University Hospital, 67091 Strasbourg, France
4Clinical Oncology Dispensary, 9/1 Zavertyaeva str., Omsk, 644013 Russia
Key words: Cytokines; C-reactive protein; Saliva; Lung cancer DOI: 10.18097/BMCRM00012 INTRODUCTION It is known that acute-phase proteins (such as C-reactive protein) and cytokines (such as IL-6) can be more easily detected in saliva than in blood serum or plasma [1-2]. Since cytokines from saliva can accumulate over time, their levels can be detected more effectively than those of cytokines in blood [3-4]. Nevertheless, a number of studies revealed difficulties in elucidating clear correlation between the cytokine levels in blood and saliva [5-7]. On the other hand, the levels of C-reactive protein in blood and saliva correlate very well and therefore its saliva concentration can be used for evaluation of the systemic inflammatory response of the body [8-9]. Saliva has a number of advantages in comparison with venous or capillary blood: non-invasive sampling and zero risk of infection while obtaining the material [10-12]. The saliva not only adequately reflects the biochemical and physiological status of a patient, but is potentially more informative medium for both clinical laboratory diagnostics and special scientific purposes [13-15]. Saliva is most widely used for the diagnostics of oral cavity diseases [16-18] including cancer [19-22]. However, in the case of lung diseases salivary cytokines were used only in diagnostics of tuberculosis [23]. Nevertheless, there is evidence that diagnosis of lung cancer may include the following potentially informative cytokines: IL-2, IL-4, IL-6, IL-8, IL-10, IL-18, tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) [24-30]. Thus the aim of this study was to evaluate the level of cytokines and CRP in saliva of patients with lung cancer in dependence of tumor sizes and distant and regional metastasis/ MATERIALS AND METHODS Study design The case-control study included 121 patients, which were divided into 3 groups: the main group (patients with diagnosed lung cancer, n = 70), the reference group (patients with non-malignant lung pathologies, n = 12) and the control group (conditionally healthy individuals, n = 39). The inclusion in groups occurred in parallel. The inclusion criteria were: the age of patients (30-75 years old), lack of any treatment including surgical, chemotherapeutic or radiation at the time of the study, the absence of signs of active infection (including purulent processes) and conduction of oral cavity sanation. The exclusion criterion was the absence of histological verification of the diagnosis.Patient recruitment and sampling The patients of the main group were examined at the Clinical Oncology Dispensary (Omsk, Russia). The patients for the control group were recruited under the routine examination at the Omsk City Ambulatory-Care Clinical Hospital no. 4. Biochemical studies were carried out at the laboratory of KhimServis LLC (Omsk, Russia). The patients of the control group underwent photofluorographic examination. The patients of the main group were hospitalized for radical surgery in the volume of lobectomy, bilobectomy, pneumonectomy, combined treatment or video-assisted thoracoscopy (VATS) for tumor biopsy. In each case, a histological verification of the diagnosis was made. The main group included patients with histologically confirmed diagnosis of lung cancer. The main group was additionally divided into subgroups according to the following characteristics: the histological type of a tumor (adenocarcinoma and squamous cell carcinoma of the lung), TNM staging and the presence/absence of distant and regional metastasis. The control group included conditionally healthy patients, who had no pulmonary pathology during routine clinical examination. The evaluation of salivary biochemical parameters of patients from the control group was performed without additional subdivision into subgroups. Test methods Samples of saliva were collected in the morning after overnight fast by spitting into sterile tubes, followed by centrifugation at 8500 g. Sample contamination with food remnants sor fluid was avoided by asking the patients to rinse their mouths, and then waiting at least 15 min before collecting the samples. Saliva samples were collected from all participants through expectoration into a sterile container over a 10-min period, after which they were immediately stored at -20 °C. The content of salivary cytokines (in pg/mL) (IL-2, IL-4, IL-6, IL-8, IL-10, IL-18, TNF-α) and CRP (in mU/L) was determined by the enzyme-linked immunosorbent assay method, using the following kits produced by “Vector-Best” (Russia): IL-2 (catalogue number A-8772), IL-4 (A-8754), IL-6 (A-8768), IL-8 (A-8762), IL-10 (A-8774), IL-18 (A-8770), TNF-α (A-8756), and CRP (A-9002). Ethical review The study was carried out in accordance with the Helsinki Declaration (adopted in June 1964 in Helsinki, Finland and revised in October 2000 in Edinburgh, Scotland) and approved at a meeting of the Local Ethics Committee of the Omsk Regional Clinical Hospital "Clinical Oncology Center" on July 21, 2016 (Protocol no. 15). Statistical analysis The statistical analysis was made using Statistica 10.0 (StatSoft, USA) and R (version 3.2.3) software using the nonparametric Wilcoxon test in dependent groups, and the Mann-Whitney U test in independent groups. Questionable results (outliers) were rejected using the Grubbs test. Results were expressed as the median (Me) and the interquartile range in the form of the 25th and 75th percentile [LQ; UQ]. Differences were considered as statistically significant at p ˂ 0.05. The statistical interrelationships were studied by means of the nonparametric correlation analysis using Spearman correlation coefficients (R). RESULTS The study included 82 patients from the Omsk Clinical Oncology Center and 39 healthy people selected as the control group. The main group consisted of 70 patients with lung cancer (squamous cell carcinoma – 27, adenocarcinoma – 32, neuroendocrine tumors – 11); the reference group consisted of 12 patients with non-malignant pulmonary pathology (tuberkulema – 4, pneumofibrosis – 3, inflammatory pseudotumor – 2, pneumonia – 3). The control group consisted of conditionally healthy patients without lung pathologies. The average age of patients was 59.2 ± 1.1 years for the main group, 56.0 ± 2.1 years for the reference group, and 52.1 ± 2.5 years for the control group. The study revealed that lung cancer caused a decrease in the levels of IL-2, IL-4, IL-18, IL-8 and TNF-α, whereas the levels of IL-6 and IL-10 increased. However, statistically significant differences were found only for IL-4 and TNF-α (Table 1). It should be noted that an increased level of IL-18 in the reference group differed from both the main group (p = 0.0472) and the control group (p = 0.0327) (Table 1).
During the next stage of the study, the main group was subdivided according to histological types of lung cancer (Table 2). For all histological types of lung cancer, the levels IL-4, IL-18, IL-8, and TNF-α were lower as compared to the control group, whereas the level of IL-6 increased. It is interesting to note that the level of IL-2 and CRP changed differently for the non-small cell lung cancer and NET (neuroendocrine tumor). An increase in the level of IL-2 was accompanied by a decrease in the CRP concentration in case of NET and vice versa for adenocarcinoma and squamous cell lung cancer.
It is interesting to consider the dynamics of concentration of the studied parameters depending on the tumor size (Table 3). In this context cytokines were subdivided into 2 groups according to the nature of concentration changes: the levels of IL-6, IL-8 and IL-18 increased with the progression of the disease, while the levels of IL-2, IL-4 and IL-10 decreased. It should be noted that the detected decrease of the cytokine level was statistically significant in the case of IL-2 (p = 0.0410), IL-4 (p = 0.0193) and IL-10 (p = 0.0485). The CRP level clearly increased along with the tumor size (p = 0.0367).
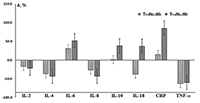
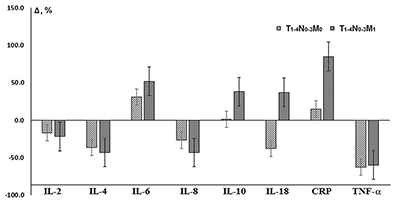
Comparison of the saliva cytokine levels in patients with the presence/absence of only distant metastasis of lung cancer showed that the dynamics of all investigated parameters was unidirectional (Fig. 1). IL-18 was the only exception: it increased compared to the controls (+37.8%) in the presence of distant metastases and decreased in their absence (-37.1%). It should be noted that despite the presence of distant metastases, the deviations of the cytokine level from control values were expressed more markedly (Fig. 1). The CRP level increased by 15.0% and 85.0% in case of the absence and presence of the distant metastases, respectively. The comparison of the salivary cytokine levels in depending on the presence/absence of regional and distant metastases (Table 4) revealed that in both cases, the levels of IL-2, IL-4, and IL-10 decreased, whereas the content of IL-6, IL-8, IL-18, and TNF-α increased; this is consistent with the dynamics of cytokine levels corresponding to the increase in the tumor size (Table 3). The level of IL-8 was higher only in case of regional metastasis (+29.7%); the levels of IL-6 and Il-18 were higher in case of regional and distant metastases (3.0 and 3.8 times higher than N0M0, respectively). The CRP and TNF-α levels were maximal under condiitons of metastases in lymph nodes (Table 4).
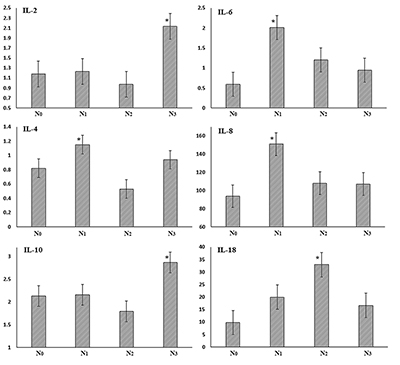
A more detailed analysis of the salivary cytokine levels in in patients with regional metastases did not reveal unidirectional changes (Fig. 2).
Therefore, despite the decrease in the average content of IL-2 and IL-10, there was a significant increase in concentration (+80.5% and +37.3% compared to N0M0, respectively) in the case of involvement of lymph nodes of mediastinum or lung root (N3M0). For IL-4, there was a local maximum at N1M0 (+40.2%) in the case of involvement of peribronchial and/or lymph nodes of the lung. Changes in the levels of IL-6 and IL-8 were similar; the maximum increase in content occurred during transition from N0M0 to N1M0 (3.4 and 1.6 times, respectively), whereas for IL-18 maximum wad observed at N2M0 (3.4-fold increase in comparison with N0M0). DISCUSSION During lung diseases, cytokines can be involved in the infectious-inflammatory process and allergic response at the level of immune mechanisms and efferent positions, which largely determines the direction, severity and outcome of the pathological process [31]. A number of cytokines can initiate and stimulate inflammatory reactions (IL-6, IL-8, IL-18, TNF-α), while others suppress them (IL-4, IL-10) [32]. IL-2 launches an immune response and activates factors involved in antiviral, antibacterial, and antitumor protection. In particular, it stimulates proliferation and activation of natural killers and cytotoxic lymphocytes. Yano et al. have shown that the level of the soluble receptor IL-2R in 24% of patients with adenocarcinoma and 40.6% of patients with squamous cell lung cancer more than 3 times exceeds the control values, whereas for the small-cell lung cancer such dependence has not been observed [24]. These authrs also found correlation between the IL-2R level and both the histological type and stage of the disease. Our research has also shown that depending on the histological type of lung cancer, the level of IL-2 changes differently: it increases in the case of NET and decreases in the case of non-small cell lung cancer. This may be attributed to the difference in nature of the tumor cells. The level of IL-2 decreases in the dynamics of the desease (Table 3). IL-4 is responsible for humoral immune response; it has local antitumor activity, inhibits production of inflammatory cytokines (IL-8, TNF-α), and regulates numerous biological processes such as proliferation, differentiation, and apoptosis in different types of cells [33]. Our study has shown that the level of IL-4 decreased in all histological types of lung cancer, but in the case of adenocarcinoma, the content of IL-4 was close to the reference values. This fact may characterize the decrease in the humoral response in the case of squamous cancer and NET of lungs, as evidenced by the decrease in the IL-4 level after the increase in the tumor size (Table 3). IL-6 plays a key role in the development of inflammation and immune response to infections or tissue damage [34]. Studies on laboratory animals have demonstrated that increased levels of serum IL-6 and vascular endothelial growth factor are associated with tumor relapse; the strongest association has been found for adenocarcinoma [26]. IL-8 causes accumulation of neutrophils, which can directly kill cancer cells, but, on the other hand, IL-8 possesses angiogenic activity and thus contributes to formation of new blood vessels and therefore to the tumor development [27]. A number of studies demonstrated that IL-6 and IL-8 expression was higher in the lung cancer tissue than in non-tumor tissue [35]. The study of IL-8 production in different cell lines of lung cancer revealed that 8 out of 13 non-small cell lines constitutively produced high levels of IL-8 [36]. Immunohistochemical analysis revealed the IL-8 staining in adenocarcinomas (22/32), squamous (12/21) and large cell (2/3) cancer, but in most samples of small cell cancer (1/22), the staining was not observed. It was also reported that elevated levels of circulating IL-8 allow predicting lung cancer 5 years before manifestations of clinical symptoms [37]. In general, the combined assay of IL-6 and IL-8 in serum can be a useful tool for determination of tactics for treatment of patients with lung cancer [26]. Despite the numerous literature data, the dynamics of salivary IL-6 and IL-8 is not uniform: regardless of the histological type of lung cancer, the level of IL-6 is higher than that for the control group, while IL-8 is lower (Table 1). However, with tumor progression, the levels of both cytokines increase in good agreement with literature data [38], at the T4N0-3M0-1 stage the level of IL-6 was 4 times higher than in control (Table 3). IL-10 plays an important role in the pathogenesis of cancer: overproduction of IL-10 increases the likelihood of tumors due to immunosuppression. On the other hand, IL-10 inhibits angiogenesis and, hence, tumor growth and metastasis [32]. It is interesting to note that in comparison with the control group, the IL-10 level in patients with squamous cell lung cancer decreases, while in adenocarcinoma and lung NET it increases. The level of IL-10 decreased with tumor progression (-34.3% at the transition from T1N0-3M0-1 to T4N0-3M0-1). Increased serum levels of IL-18 in patients with different forms of cancer correlate with the progression of the disease and the development of metastasis. On one hand, IL-18 enhances the expression of Fas-ligand on natural killers and T-lymphocytes, inhibits growth of blood vessels in tumor tissue. On the other hand, it stimulates formation of cell adhesion molecules, such as IL-8, which can trigger tumor metastasis [32]. It is known that the level of IL-18 in the sputum of lung cancer patients is significantly higher than corresponding control values [28]. The authors suggest to use correlation of vascular endothelial growth factor and the IL-18 level for immunologic differentiation between small-cell and non-small cell lung cancer. The salivary IL-18 level decreases in lung cancer; however, it grows depending on the stage of the disease (Table 3). It is known that the level of TNF-α directly depends on the severity of endogenous intoxication [39]. However, according to the obtained results, the level of TNF-α in lung cancer was 2.67 times lower than for the control group (p = 0.0060). This fact can be explained by the large spread in the TNF-α values in the control group, and also by a significant impact of the periodontium status on this parameter, which must be taken into account in further studies [18]. The comparison of the cytokine level dynamics under condiitons of lung cancer of lung cancer and pulmonary inflammatory disease shows that the levels of anti-inflammatory cytokines change similar in both cases. The level of IL-2 is lower in case of lung cancer (-31.0%) and the level of IL-4 is lower in the case of inflammatory diseases (-36.4% in comparison with the control group). Data on the content of IL-6 for the comparison group were not obtained, but other proinflammatory cytokines (IL-8, IL-18 and TNF-α) decreased in lung cancer and increased in inflammatory diseases. However, the comparison of the content of proinflammatory cytokines during the disease progression (stage T4N0-3M0-1) reveals that in this case the difference between the main group and the reference group reduces. This fact can be associated with more pronounced inflammation that accompanies the tumor process. CRP is the most sensitive and quick indicator of tissue damage of different origin (inflammation, necrosis, neoplastic process); it reflects the activity and stage of the disease [40-41]. During the acute phase response, CRP is secreted by hepatocytes (to a lesser extent by renal cells, endothelial and vascular unstriped muscles, monocytes, and neutrophils) in response to tissue damage and activity of various cytokines (IL-1, IL-6 and TNF-α) [40,42]. It is therefore quite natural that the CRP concentration is higher in patients with lung cancer and evenly grows with the disease progression (Tables 2, 3). However, taking into account the histological type of the tumor this shows that the CRP level increases in the case of non-small cell lung cancer and decreases in the case of NET. The CRP level in the reference group is intermediate between the main and control groups. A recent meta-analysis has demonstrated that a high basal level of blood CRP is associated with a worse prognosis for surgical treatment of lung cancer [42]. Thus, it can be assumed that the preoperative level of CRP can be used as a predictive factor at the early stages of lung cancer, alone or in combination with other features of the tumor or the patient [26,43-44]. One of the factors that determine survival rates for lung cancer is the frequency of lymphatic metastasis. It has been demonstrated that the maximum increase in the level of proinflammatory cytokines corresponds to the involvement of peribronchial and/or lymph nodes of the lung root (IL-6, IL-8), as well as the subcarinal lymph nodes or mediastinum (IL-18, TNF-α). This fact is consistent with the literature data on different expression of cytokines of the lymph node with metastases and the benign lymph node; in particular the levels of IL-6 and TNF-α were signifujcantly higher in malignant lymph node samples than in benign samples [45-46]. On the other hand, the maximum increase in the levels of IL-2 and IL-10 corresponds to the high incidence of regional metastasis, which may be caused by the immunosuppression on these stages of the disease. CONCLUSIONS Our data have shown that the cytokine levels in the saliva of lung cancer patients and healthy individuals do not differ statistically. It has been demonstrated that the content of IL-2 and IL-4 in saliva reduces in case of both lung cancer and inflammatory lung diseases, whereas the levels of IL-18, IL-8 and TNF-α decrease in lung cancer and increase in non-tumorous pathologies. Tumor progression causes an increase in the content of proinflammatory cytokines (IL-6, IL-8, IL-18, TNF-α), whereas the levels of IL-2, IL-4 and IL-10 decrease. The increased levels of C-reactive protein and tumor markers in lung cancer dynamics have been observed; however, insignificant differences from inflammatory lung diseases have been detected. The recognzied dynamics of the saliva cytokines level undre conditions of distant and regional metastases opens the prospect of using these parameters for monitoring the treatment process and controlling the relapse occurrence. REFERENCES
|