Superoxide Generation by Nicotinamide Coenzymes
Institute of Theoretical and Experimental Biophysics, ul. Institutskaya 3; Pushchino, 142290 Russia; *e-mail: sirotatv@rambler.ru
Keywords: superoxide; nitro blue tetrazolium; diformazan; nicotinamide coenzymes; NADPH; NADP; NADH; NAD
DOI:10.18097/BMCRM00188
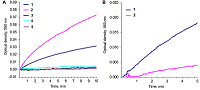
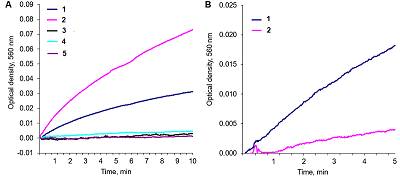
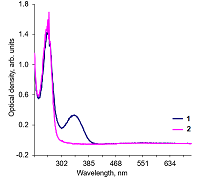
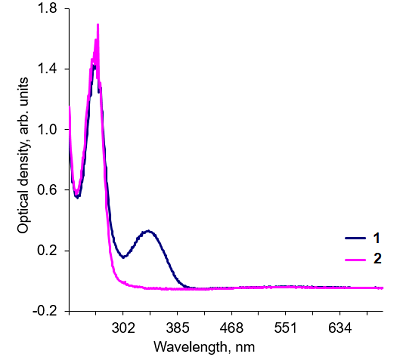
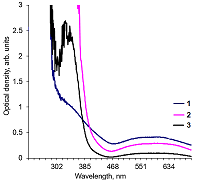
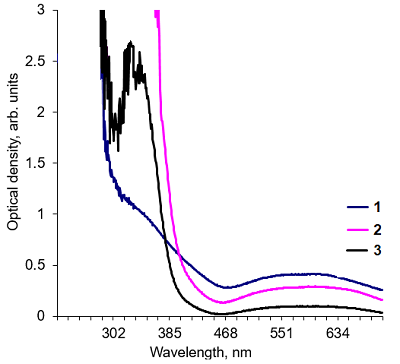
Alkaline solutions of nicotinamide coenzymes can generate superoxide radicals. Their formation was registered by reduction of nitroblue tetrazolium (NBT) present in the buffer with formation of diformasan. Inhibition of diformazan formation occurs when superoxide dimutase (SOD) is added to the system, thus confirming generation of О2─●. The highest superoxide generating activity was observed with NADPH. In the case of NADPH and NADH, the rate of superoxide generation was significantly lower (by approximately 50%). No О2─● was detected when NAD was used under the same conditions and in the same time; however, 4 h later, diformasan was detected in the same sample. The superoxide generating activity decreased in the following order: NADPH > NADH ≥ NADP > NAD. Other compounds tested (adenosine, ADP and ATP) did not generate superoxide radicals even after prolonged incubation. In a cell, where a local changes in the pH of the environment are possible, nicotinamide coenzymes can be potential sources of О2─● and thus participate in cell signaling. A change in pH can initiate this process.
|
CLOSE

|
Table 1.
Superoxide generating activity of the studied compounds.
|
FUNDING
The work was carried out within the framework of the State assignment ITEB RAS No. 075-01027-22-00
REFERENCES
- Ray, P. D., Huang, Bо-W., Tsuji, Y. (2012) Reactiv oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal, 24(5), 981-990. DOI
- Zenkov, N.K., Kolpakov, A.R., Menshchhikova, E.B. (2015) Keap1/Nrf2/are redox-sensitive system as a phagmacological target in cardiovascular diseases. Sibirskii nauchnii meditsinskii zhornal, 35(5), 5-25.
- Valko, M., Leibfritz, D., Moncol, J., Cronin, M.T.D., Mazur, M, Telser, J. (2007) Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology, 39(1), 44-84. DOI
- Wang, Y., Branicky, R., Noë, A., Hekimi, S. (2018) Superoxide dismutases: dual roles incontrolling ROS damage and regulating ROS signaling. Journal of Cell Biology, 217, 1915-1928. DOI
- Oxidative Stress Reduction, Redox Homeostasis & Antioxidants Retrived November 16, 2021, from www.isanh.net
- Weihai, Y. (2006) NAD+ and NADH in cellular functions and cell death. Frontiers in Bioscience, 11, 3129 – 3148. DOI
- Pollak, N., Dölle, C., Ziegler, M. (2007) The power to reduce: pyridine nucleotides--small molecules with a multitude of functions. The Biochemical journal, 402(2), 205-218. DOI
- Alhasan, R., Njus, D. (2008) The epinephrine assay for superoxide: Why dopamine does not work. Analytical Biochemistry, 381(1), 142-147. DOI
- Zenkov, N.K., Menshchikova, E.B. (1993) Activated oxygen metabolites in biological systems. Uspechi sovremnoi biologii, 113(3), 286-296.
- Sirota, T.V., Sirota, N.P. (2022) On the mechanism of oxygen activation in chemical and biological systems. Biophysics, 67(1), 1–7. DOI
- Nishikimi, M., Appaji, N., Yagi, K. (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun., 46(2), 849-854. DOI
- Ponti, V., Dianzani, M.U., Cheeseman, K., Slater, T. F. (1978) Studies on the reduction of nitroblue tetrazolium chloride mediated through the action of NADH and phenazine methosulphate. Chemico-Biological Interactions, 23(3), 281-291. DOI
- Picker, S.D., Fridovich, I. (1984) On the mechanism of production of superoxide radical by reaction mixtures containing NADH, phenazine methosulfate, and nitroblue tetrazolium. Archives of Biochemistry Biophysics, 228, 155-158. DOI
- Rao, U. M. (1989) Source of superoxide anion radical in aerobic mixtures consisting of NAD[P]H, 5-methylphenazinium methyl sulfate and nitroblue tetrazolium chloride. Free Radicals Biology and Medicine, 7(5), 513-519. DOI
- Altman, F.P. (1976) Tetrazolium salts and formazans. Progress in Histochemistry and Cytochemistry, 9(3), 1-52. DOI
- Sirota, T.V. (2020) A сhain reaction of adrenaline autoxidation is a model of quinoid oxidation of catecholamines. Biophysics, 65 (4), 548. DOI
- Spravochnik po biokhimii (1971) (ed. F.L. Kalinin, V.P. Lobov, V.A. Zhidkov), pp. 320-322, Naukova Dumka, Kyiv.
- Hayyan, М. Hashim, M.A., AlNashef, I.M. (2016) Superoxide ion: Generation and chemical implications. Chemical Reviews, 116(5), 3029–3085. 10.1021/acs.chemrev.5b00407 DOI
- Berezhnov, A.V., Soutar, M.P., Fedotova, E.I., Frolova, M.S., Plun-Favreau, H., Zinchenko, V.P., Abramov, A. Y. (2016) Intracellular pH modulates autophagy and m itophagy.The Journal of biological chemistry, 291(16), 8701- 8708. DOI