COMPARISON OF THE EFFICIENCY OF DENDRITIC CELL MATURATION UPON STIMULATION WITH BACTERIAL LIPOPOLYSACCHARIDE OR TUMOR NECROSIS FACTOR ALFA
1Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: biocell@inbox.ru
2Moscow Gymnasium in the Southwest No. 1543 named after Yu.V. Zavelsky,
3 build. 5, 26 Baku Komissarov, Moscow, 119571 Russia
3A.V. Vishnevsky Institute of Surgery, 27 Bolshaya Serpukhovskaya, Moscow, 117997 Russia
Keywords:dendritic cells; induced maturation; tumor necrosis factor; lipopolysaccharide; cell-based vaccines
DOI:10.18097/BMCRM00309
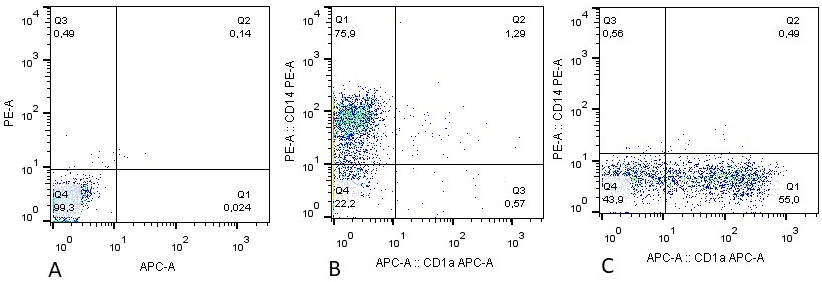
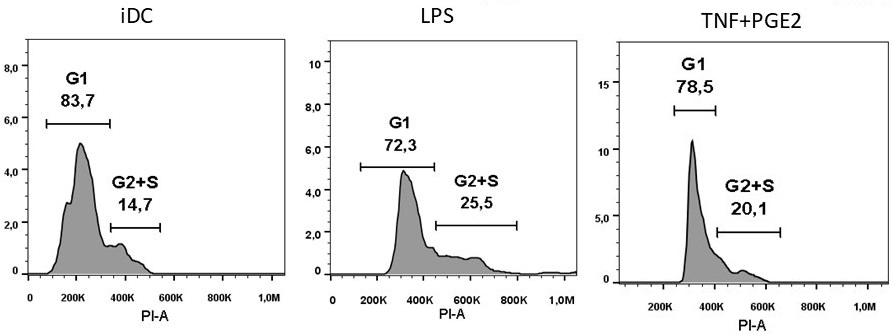
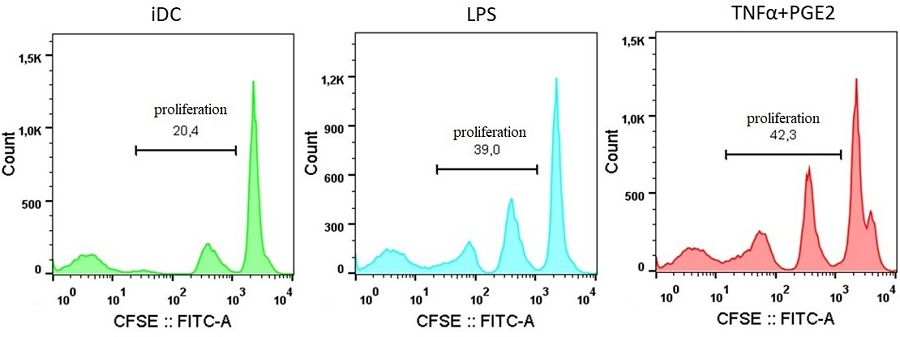
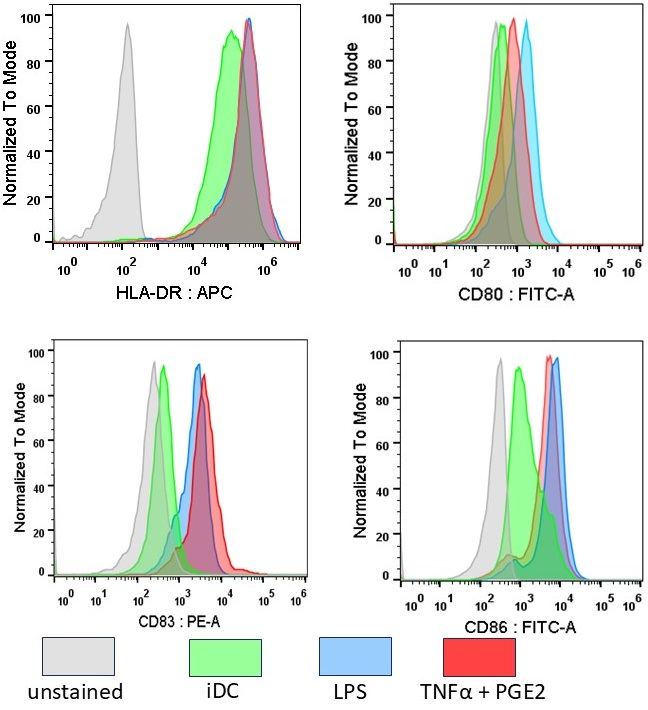
Dendritic cells (DCs) are the professional antigen-presenting cells capable of presenting antigens to T-lymphocytes, thereby initiating the primary immune response. The unique immunological properties of this cell population make their use as a cellular vaccine relevant for the treatment of oncological and chronic infectious diseases. A critical stage in obtaining DCs with immunostimulatory properties is their maturation. To compare the effectiveness of alternative methods for stimulating maturation, DCs were obtained in vitro from peripheral blood monocytes through their induced differentiation in the presence of the cytokines GM-CSF and IL-4. As biochemical stimuli for inducing maturation, bacterial lipopolysaccharide (LPS) or tumor necrosis factor alpha combined with prostaglandin E2 (TNFα+PGE2) were used. No significant differences were found in the ability of these factors to stimulate the expression of HLA-DR and costimulatory molecules (CD80, CD83, CD86). The use of alternative maturations did not lead to differences in the ability of DCs to stimulate the proliferation of allogeneic lymphocytes. At the same time, there were morphological signs indicating the ability of LPS to stimulate differentiation into macrophages.

|
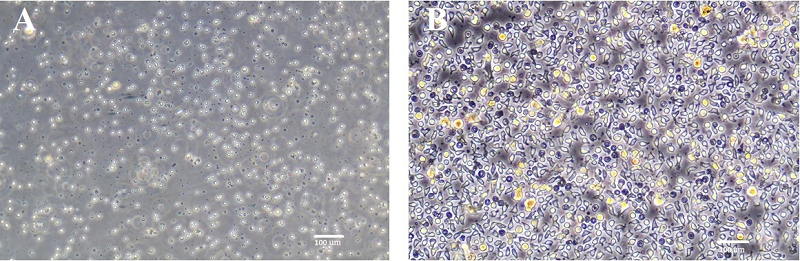
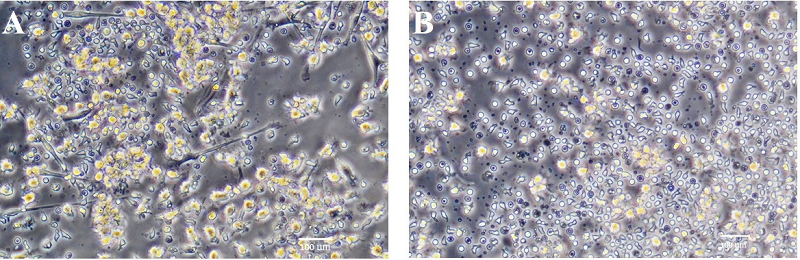
Figure 3.
Dendritic cell cultures after induction of their maturation using LPS (A) or TNFα + PGE2 (B). Phase-contrast light microscopy.
|
FUNDING
This work was performed within the framework of the Program of Fundamental Scientific Research in the Russian Federation for the long-term period (2021-2030) No. 122022800499-5.
REFERENCES
- Tsapogas, P., Mooney, C. J., Brown, G., Rolink, A. (2017) The cytokine Flt3- ligand in normal and malignant hematopoiesis. Int J Mol Sci., 18(6), 1115. DOI
- Segura E. (2025) Monocyte-Derived Dendritic Cells: An Updated View on an Old Concept. Immunol Rev., 336(1), e70075. DOI
- Christopherson, K., Hromas, R. (2001) Chemokine regulation of normal and pathologic immune responses. Stem Cells, 19, 388-396. DOI
- Karalkin, P. A., Lupatov, A. Y., Yarygin, K. N. (2009) Endocytosis of microand nanosized particles in vitro by human dendritic cells. Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology, 3(4), 410-416. DOI
- Jarrossay, D., Napolitani, G., Colonna, M., Sallusto, F., Lanzavecchia, A. (2001) Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol., 31, 3388- 3393. DOI
- Figdor, C.G., van Kooyk, Y., Adema, G. J. (2002) C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol., 2, 77-84. DOI
- Masten, B. J., Yates, J. L., Pollard Koga, A. M., Lipscomb, M. F. (1997) Characterization of accessory molecules in murine lung dendritic cell function: roles for CD80, CD86, CD54, and CD40L. Am. J. Respir. Cell. Mol. Biol., 16, 335-342. DOI
- Moussion, C., Delamarre, L. (2024) Antigen cross-presentation by dendritic cells: A critical axis in cancer immunotherapy. Semin Immunol., 71, 101848. DOI
- Sander, J., Schmidt, S.V., Cirovic, B., McGovern, N., Papantonopoulou, O., et al. (2017) Cellular differentiation of human monocytes is regulated by timedependent interleukin-4 signaling and the transcriptional regulator NCOR2. Immunity, 47 (6), 1051-1066 DOI
- Mahnke, K., Schmitt, E., Bonifaz, L., Enk, A. H., Jonuleit, H. (2002) Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol., 80(5), 477-483. DOI
- Brigl, M., Brenner, M. B. (2004) CD1: antigen presentation and T cell function. Annu. Rev. Immunol., 22(1), 817-890. DOI
- Jonuleit, H., Kuhn, U., Muller, G., Steinbrink, K., Paragnik, L., Schmitt, E., Knop, J., Enk, A.H. (1997) Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol., 27, 3135-3142. DOI
- Lupatov, A. Yu., Karalkin, P. A., Moldaver, M. V., Burunova, V. V., Poltavtseva, R. A., Gabibullaeva, Z. G., Pavlovich, S. V., Yarygin, K. N., Sukhikh, G. T. (2011) Bone marrow mesenchymal stem cells suppress differentiation of allogeneic dendritic cells in vitro and do not affect their maturation. Immunologiya, 32(3), 122-127.
- Winzler, C., Rovere, P., Rescigno, M., Granucci, F., Penna, G., Adorini, L., Zimmermann, V. S, Davoust, J., Ricciardi-Castagnoli, P. (1997) Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med., 185(2) 317-328. DOI
- Bourque, J., Hawiger, D. (2023) Life and death of tolerogenic dendritic cells. Trends Immunol., 44(2), 110-118. DOI
- Sansom, D. M. (2000) CD28, CTLA-4 and their ligands: who does what and to whom? Immunology, 101(2), 169-177. DOI
- Cao, W., Lee, S. H., Lu, J. (2005) CD83 is preformed inside monocytes, macrophages and dendritic cells, but it is only stably expressed on activated dendritic cells. Biochem J., 1, 385(Pt 1), 85-93. DOI
- Kawai, T., Akira, S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol., 11(5), 373-384. DOI
- Horiuchi, T., Mitoma, H., Harashima, S., Tsukamoto, H, Shimoda, T. (2010) Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford), 49(7), 1215-1228. DOI
- Akira, S, Uematsu, S., Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell, 124(4), 783-801. DOI
- Sallusto, F., Lanzavecchia, A. (1994). Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/ macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha, Journal of Experimental Medicine,
- Jonuleit, H., Kühn, U., Müller., G., Steinbrink, K., Paragnik L., Schmit, E., Knop, J., Enk, A.H. (1997) Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol., 27(12), 3135-3142. DOI
- Lichtenegger, F.S, Mueller, K., Otte, B., Beck, B., Hiddemann, W., Schendel, D.J, Subklewe, M. (2012) CD86 and IL-12p70 are key players for T helper 1 polarization and natural killer cell activation by Toll-like receptor-induced dendritic cells. PLoS One, 7(9), e44266. DOI
- Dohnal, A. M., Witt, V., Hügel, H., Holter, W., Gadner, H., Felzmann, T. (2007) Phase I study of tumor Ag-loaded IL-12 secreting semi-mature DC for the treatment of pediatric cancer. Cytotherapy, 9(8), 755-770. DOI
- Boullart, A. C., Aarntzen, E. H., Verdijk, P., Jacobs, J. F., Schuurhuis, D. H., Benitez-Ribas, D., Schreibelt, G., van de Rakt, M. W., Scharenborg, N. M., de Boer, A., Kramer, M., Figdor, C. G., Punt, C. J., Adema, G. J., de Vries, I. J. (2008) Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer Immunol Immunother., 57(11), 1589- 1597. DOI